2. 中国科学院大学, 北京 100049;
3. 莱斯特大学遗传学和基因组生物学系, 莱斯特 LE1 7RH, 英国;
4. 华南国家植物园, 广州 510650
2. China University of Chinese Academy of Sciences, Beijing 100049, China;
3. Department of Genetics and Genome Biology, University of Leicester, Leicester, LE1 7RH, UK;
4. South China National Botanical Garden, Guangzhou 510650, China
蛋白激酶是细胞功能的关键调节因子。类受体激酶(receptor-like kinases, RLKs)是植物中数量最多的蛋白激酶家族,约占激酶总数的60%[1],RLKs通过细胞表面的受体来感知外部信号,传输到下游效应分子,启动信号传导,还通过磷酸化修饰来激活或抑制其他靶蛋白,参与调控细胞的生长、分化、免疫应答、神经信号传导、细胞凋亡等生命过程[2]。因此,RLKs在植物应对生物、非生物胁迫方面发挥关键作用。
根据胞外区氨基酸序列的差异,RLKs分为10类:凝集素RLKs (lectin RLKs; 凝集素lectin,来源于拉丁文legere,意思是选择)、富含亮氨酸重复区RLKs (leucine-rich repeats, LRR-RLKs)、具有S结构域RLKs (RLKs with S-domain)、与细胞壁相连的RLKs (wall-associated kinases, WAK-like RLKs)、富含脯氨酸的类伸展素RLKs (proline-rich extensin-like receptor kinases, PERKs)、几丁质酶相关RLKs (chitinaserelated RLKs)、类表皮生长因子型RLKs (epidermal growth factor-like, EGF-like RLKs)、类肿瘤坏死因子型RLKs (tumor-necrosis factor receptor-like, TNFR-like RLKs)、类PR5型RLKs (pathogenesis related protein-5 like receptor kinases, PR5K)、以及含有Malectin-like结构域的长春花RLKs (Catharanthus roseus, CrRLKs)[3]。其中,凝集素类受体激酶(lectin receptor-like kinases, LecRLKs) N端的凝集素结构域能够与碳水化合物可逆结合[4]。对LecRLKs的研究主要集中在模式植物中,在水稻(Oryza sativa)中发现173个LecRLKs家族成员,拟南芥(Arabidopsis thaliana)中发现75个[5]。近年来, 随着基因组测序技术的进步和功能基因组学研究的深入,LecRLKs在植物生长发育和响应外界环境中的作用逐渐受到关注。
本文概述了植物凝集素类受体激酶的研究进展。总结LecRLKs的结构特征和分类,系统阐述LecRLKs在植物生物学中的功能,聚焦LecRLKs在植物根、茎、叶、花和种子等器官中的发育调控功能,以及参与生物、非生物胁迫的重要功能。旨在为LecRLKs基因家族的全基因组鉴定、演化历史、功能验证等研究提供证据,并为LecRLKs基因家族的定向育种提供理论支持。
1 凝集素类受体激酶的结构和分类 1.1 凝集素类受体激酶结构组成凝集素类受体激酶主要由胞外凝集素结构域、跨膜结构域和胞内丝氨酸/苏氨酸激酶结构域组成。凝集素结构域参与信号的识别,可与糖类分子可逆结合,胞外凝集素受体结构域的差异,是LecRLKs分类的依据[6]。
跨膜结构域一般具有18~25个氨基酸,序列保守性低[7]。对烟草LecRLKs结构域的生物信息学研究发现,少数LecRLKs不含跨膜结构域[8]。在杨属(Populus)植物中,存在2~3个跨膜结构域的凝集素类受体蛋白激酶[9]。这说明跨膜结构域并非是必不可少的结构域元件,少数凝集素类受体激酶不存在跨膜结构域。
胞内激酶结构域,一般由250~300个氨基酸构成,序列保守性高[10]。N端带有GxGxxG序列,影响与核苷酸的结合,C端则含有43~66个氨基酸, 对激酶的催化活性至关重要[7],该区域被认为是下游信号分子相互作用的调控区域[6]。LecRLKs的激酶结构域,包括DIKPAN和GT(FIL)GYIAPE序列, 属于丝氨酸/苏氨酸激酶[11]。拟南芥的LecRK-Ⅴ.5,ATP (adenosine 5′-triphosphate)结合位点和催化位点包括天冬氨酸、赖氨酸和天冬酰胺三联体[12],该结构域具有磷酸化位点,负责外部信号传递。酶动力学研究表明,部分二价金属离子,如Mn2+和Mg2+,可促进激酶结构域的自身磷酸化和激酶活性,其中Mn2+和Mg2+的促进作用,强于Ca2+和Zn2+[12–13]。
1.2 凝集素类受体激酶分类根据胞外凝集素结构的不同,LecRLK分为3个亚家族:L-LecRLKs、G-LecRLKs和C-LecRLKs[6] (图 1)。其中L和G型是植物特有的,C型在哺乳动物中常见[13]。对拟南芥[11]和蒺藜苜蓿(Medicago truncatula)[14]的研究表明,凝集素类受体激酶分子量高于预测值,可能存在翻译后修饰,如糖基化[6]。
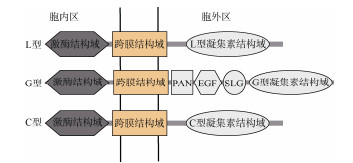
|
图 1 凝集素类受体激酶的结构和分类(改绘自彭小群等[7])。PAN: 纤溶酶原基序; EGF: 表皮生长因子; SLG: S位点糖蛋白结构域。 Fig. 1 Classification and structure of LecRLKs (redraw from Peng X Q et al.[7]). PAN: Plasminogen/apple/nematode; EGF: Cysteine-rich epidermal growth factor; SLG: S-locus glycoprotein domain. |
L型凝集素类受体激酶,其N端具有豆科植物凝集素(legume-lectin)结构域,通过与疏水配体之间互作,感知并传导外部信号[7]。legume-lectin结构域约有250个氨基酸,呈典型β-三明治(β-sandwich)折叠,包含糖类结合位点和疏水腔结构,可与疏水配体结合,如复合糖、植物激素和病原体相关分子模式(pathogen-associated molecular patterns, PAMPs)[15], 该结构域包含Ca2+和Mn2+,具有稳定糖类结合位点的功能[7]。有研究表明,许多L型凝集素结构域中,糖类结合位点、Ca2+和Mn2+结合位点的氨基酸序列保守性低[11]。
1.2.2 G型LecRLKsG型凝集素类受体激酶,曾被称为B型和S型LecRLKs[13, 16],含有GNA (Galanthus nivalis agglutinin, 雪花莲凝集素)结构域[6],具有12条β-链组成的β-酒桶(β-barrel)结构,对α-d甘露醇(α-d mannose)具有亲和力[15]。其胞外结构域还包括S-locus糖蛋白(S-locus glycoprotein, SLG)结构域、表皮生长因子(cysteine-rich epidermal growth factor, EGF)结构域和纤溶酶原结构域(plasminogen/apple/nematode, PAN)[5]。SLG结构域参与十字花科植物的自交不亲和;EGF基序富含半胱氨酸,参与二硫键的形成; PAN结构域参与蛋白质之间、蛋白质与碳水化合物之间的互作[6]。G-LecRLKs在植物-微生物相互作用和免疫反应中起重要作用,识别真菌和致病线虫(Meloidogyne spp.) [17–18],如PtLecRLK1促进真菌的根定植,介导真菌与植物的共生关系[19]。
1.2.3 C型LecRLKsC型凝集素类受体激酶,具有钙依赖型(calcium-dependent)凝集素结构域,在植物中存在较少,且作用不明确,仅在拟南芥、水稻、毛果杨(Populus trichocarpa)和桉树(Eucalyptus robusta)中各发现1个C型凝集素类受体激酶[5, 13]。C-LecRLKs在哺乳动物中比较常见,主要作用是参与病原体识别、免疫应答以及抗感染防御等[16]。
2 凝集素类受体激酶的发现凝集素类受体激酶定位于细胞质膜上,识别病原体、共生体及胁迫信号,协调细胞生长,参与植物发育、胁迫的应答及先天免疫过程[15]。凝集素类受体激酶识别特定的配体,启动信号通路[20],检测环境变化以及调控生长与防御的平衡,在增强植物的适应性中发挥关键作用[9]。Walker等[21]首次在玉米(Zea mays)中鉴定到凝集素类受体激酶ZmPK1, 属于G型LecRLKs,其胞外结构域参与花粉和柱头之间的识别。Herve等[22]在拟南芥中,鉴定到第1个L型凝集素类受体蛋白激酶Ath.LecRK1,其胞外结构域与豆科植物凝集素家族的碳水化合物结合蛋白同源。随着基因组测序技术的发展,LecRLKs被系统归类为RLKs的1个亚家族,随后学者们又对LecRLKs的结构、分类以及功能开展系统研究[6]。
目前,在单子叶植物和双子叶植物中都鉴定到LecRLKs成员,如水稻[5]、小麦(Triticum aestivum)[23]、烟草(Nicotiana benthamiana)[8]和拟南芥[5]等,根据已鉴定该家族的植物中的凝集素类受体激酶数量统计,G型LecRLKs多于L型和C型(拟南芥除外),各物种中只报道了少数C型LecRLKs成员,目前对凝集素类受体激酶的研究主要集中在拟南芥和水稻等模式植物上(表 1)。与单子叶植物相比,双子叶植物的LecRLKs同源性较高[11],在人类和酵母基因组中则缺乏凝集素类受体激酶的同源物[16]。
| 表 1 植物中LecRLKs基因家族成员 Table 1 Identified LecRLKs gene family members in plants |
凝集素类受体激酶参与调控植物生长发育的诸多生物过程,如花粉发育、种子萌发、衰老、纤维发育、株高等。调节雄性减数分裂和胞质分裂的进程,以及微孢子发生过程中的绒毡层细胞程序性死亡,参与激素调控的种子萌发过程。水稻、拟南芥、番茄(Lycopersicon esculentum)和花生(Arachis hypogaea)等植物中的凝集素类受体激酶均参与植物生长发育,且大部分的调控机制尚不明晰,其中1个重要的通路是植物激素信号响应[15]。本文总结了参与植物生长发育调控的凝集素类受体激酶基因(表 2)。
| 表 2 参与生长发育的凝集素类受体激酶基因 Table 2 The LecRLK genes involved in growth and development of plants |
在水稻中,AP1、OsLecRK-S.7、OsLecRK5、OsDAF1这4个L型LecRLKs等位基因的突变体均表现出配子体缺陷[34]。AP1在花粉成熟过程中参与淀粉积累,AP1的突变下调与花粉淀粉代谢途径有关的蛋白质的磷酸化,影响花粉内壁孔径的形成[34]。OsLecRK-S.7参与水稻花粉外壁的形成,过表达OsLecRK-S.7会导致水稻花序发育迟缓,质谱分析结果表明,其激酶结构域对信号转导至关重要,而OsLecRK-S.7启动发育信号的机制仍不清楚[35]。OsLecRK5磷酸化胼胝质合成酶UGP1正向调节水稻中的胼胝质生物合成[36]。水稻OsINP1可以招募OsDAF1到萌发孔位置,调节水稻孔径模式的形成,OsINP1突变体的花粉环面消失乃至雄性不育[37]。水稻LecRK7基因突变体,表型为花粉块粘连,因此,推测LecRK7基因可能在花粉发育前期起作用[38]。
水稻OslecRK (G型)基因参与种子萌发和植物先天免疫[5],研究表明OslecRK通过其激酶结构域与体内肌动蛋白解聚因子互作,增加α-淀粉酶基因的表达,进而刺激种子发芽和防御相关基因的表达[39]。OsSRK1 (L型)基因在水稻茎和节间高表达,参与节间伸长和植株长高,推测该激酶与赤霉素受体GID1相互作用,正向调节植物高度[40]。水稻OsSIK2 (G型)延缓叶片衰老,促进叶片出苗,提高植物对盐胁迫的耐受性[41]。PWL1 (G型)突变体表型为水稻早衰[42]。
拟南芥有45个L型LecRLKs,其中参与生长发育的较少,如SGC基因突变体,表型为花粉粒粘连、雄性不育,SGC可能结合寡糖,在花粉发育事件的上下游起着连接作用,保证花粉发育的持续进行[43]。拟南芥LecRKA4家族的LecRKA4.1/LecRKA4.2/ LecRKA4.3基因,位于第5号染色体上,编码膜相关蛋白,RNA干扰转基因实验表明,它们都是脱落酸(abscisic acid, ABA)反应的负调节因子,突变体植株种子发育延缓[44]。
LecRK-VIII.2基因控制种子产量。Xiao等[45]构建过表达株系实验,认为LecRK-VIII.2基因可促进种子增大,减少种子结籽数,还可促进根、茎、叶的生长,减少种子中光合产物的分配;LecRK-Ⅷ.2上调有丝分裂原活化蛋白激酶MPK6的磷酸化水平,协调长角果数量、种子大小和数量的平衡。LecRK-VIII.2基因参与调控拟南芥下胚轴生长,抑制植物开花[46]。报告基因检测表明,LecRK-V.5启动子中存在衰老调控的顺式作用元件,LecRK-V.5基因主要在衰老子叶和叶片中表达,与叶绿素含量下降有负相关关系[47]。
Micol-Ponce等[48]鉴定到1个SlG-LecRK-II.9 (G型)基因,转录组学分析表明,SlG-LecRK-II.9协调番茄花粉小孢子母细胞减数分裂过程、DNA修复和胞质分裂的信号传导,SlG-LecRK-II.9基因缺失导致绒毡层程序性细胞死亡,造成小孢子败育。黄若兰[49]将花生AhLecRK9 (L型)转基因到拟南芥中, 过表达植株的抽薹时间提前、种子更大,说明AhLec-RK9参与种子增大的过程。小麦TaLecRK-IV.1 (L型)基因正向调节植物高度,在CI12633小麦系中沉默该基因,导致小麦植株矮小[50]。据报道,陆地棉(Gossypium hirsutum) L型凝集素类受体激酶GhlecRK参与纤维发育[51]。
Haider等[32]通过顺式作用元件分析,认为黄瓜(Cucumis sativus)中L型和G型凝集素类受体激酶参与植物器官的发育,转录组学分析表明,L型LecRLKs基因比G型LecRLKs基因具有更敏感的胁迫响应性。基因组学鉴定表明,黄瓜LecRLKs家族有6个与发育相关的顺式作用元件,其中5个与种子发育相关,表明该基因家族在种子发育中发挥作用[53]。转录组学分析表明,在豆梨(Pyrus bretschneideri)的根、茎、叶、花和果实中均检测到LecRLKs基因表达[26];小麦根中LecRK-V的表达升高[54];毛果杨中LecRLKs在花序中表达,推测LecRLKs与繁殖器官的生长发育有关[9]。
4 凝集素类受体激酶参与对外界胁迫的响应凝集素类受体激酶是植物细胞壁表面的模式识别受体(pattern recognition receptor, PRR),结构域的蛋白质序列高度可变,使LecRLKs识别病原体、真菌在内的多种生物体,触发胼胝质沉积、MAPK激活、钙内流、产生活性氧(reactive oxygen species, ROS)和水杨酸(salicylic acid, SA)积累等免疫过程, 在植物与真菌共生中发挥积极作用。植物激素也是非生物胁迫反应中的重要参与者,如乙烯信号通路和乙烯受体蛋白能够调控LecRK-I.3基因在盐胁迫下的表达。
4.1 参与非生物胁迫响应LecRLKs参与植物对非生物胁迫的反应,抵抗非生物胁迫,例如盐胁迫、低温胁迫、干旱胁迫和机械损伤等(表 3)。LecRLKs通过自身磷酸化或磷酸化其他蛋白质,将信号传递到下游通路,SIT1在盐胁迫过程中被激活,磷酸化下游效应子(MAPK3/6),触发乙烯信号诱导的ROS积累,增强植物的敏感性[10]。
| 表 3 参与非生物胁迫的凝集素类受体激酶基因 Table 3 LecRLK genes involved in the abiotic stress |
盐胁迫最普遍的非生物胁迫之一,高盐环境植物根系周围的水势降低,导致离子平衡紊乱、细胞膜受损、植物光合效率降低[55],拟南芥中的L型LecRK-I.3受乙烯受体蛋白和乙烯信号通路的调控,参与盐胁迫响应[56];LecRK-V.2基因在种子萌发阶段表达[57];AtLPK1基因在高盐环境下,正向调节盐胁迫响应[58];LecRKIII.2基因提高拟南芥幼苗对盐胁迫的耐受性[59]。野大豆(Glycine soja)的GsSRK和GmLecRLK是盐胁迫响应基因,GsSRK在野大豆中受盐胁迫和ABA诱导,调控植物对盐和干旱胁迫的耐受性[60];过表达野大豆GmLecRLK基因,增强了清除ROS的能力,提高耐盐性[61]。PsLecRLK基因在豌豆(Pisum sativum)根中积累,提高豌豆的耐盐性,防止ROS积累和膜损伤[62]。研究表明,水稻的L型凝集素类受体激酶SIT1,通过正向调控乙烯生成,负调控耐盐性。在拟南芥中,SIT1通过积累ROS使盐胁迫下植物的存活率降低[10]。豆梨PbLEK066基因在盐胁迫下,表达量显著升高[63]。盐胁迫下欧李(Cerasus humilis)的ChLecR-LKs基因显著上调[29]。欧洲甜樱桃(Prunus avium)中的PaLectinL7 (L型)通过调节木质素沉积,从而影响植物耐盐性[64];功能研究表明,在拟南芥中过表达欧洲甜樱桃L型凝集素类受体激酶PaLectinL16, 增强植株的耐盐性,此外,欧洲甜樱桃砧木Gisela 6转基因的PaLectinL16过表达植株,增强ROS清除酶的活性,提高了植物耐盐性[33]。花生AhLecRK9 (L型)过表达植株,增加植物对盐胁迫的敏感性[49]。
凝集素类受体激酶参与植物对低温胁迫的响应。拟南芥的LecRK-S.7基因的启动子区含有低温胁迫应答元件[65]。拟南芥LecRKIII.2基因在低温处理后呈先上升后下降的趋势[59]。豌豆PsLecRLK基因在低温条件下的表达量增加[62]。黄丝瓜藓(Pohlia nutans)的PnLecRLK1基因与低温耐受性增强有关,拟南芥PnLecRLK1基因的过表达植株对低温胁迫的耐受性增强[66]。
凝集素类受体激酶参与干旱胁迫响应,大豆GsSRK基因在干旱胁迫下诱导表达[60];欧李LecRLKs家族的9个基因在强干旱条件下,根中的LecRLKs表达量升高,在轻微干旱条件下,其中3个基因在根中表达量升高,说明LecRLKs对不同干旱胁迫的响应存在差别[29]。黄若兰[49]将花生AhLecRK9转基因到拟南芥中,过表达植株在干旱处理后的根相对于野生型更长,表明AhLecRK9增强了根的耐旱性[49]。
植物抵御机械损伤涉及ROS的产生、钙信号传导和脂质代谢等过程,拟南芥LecRK-V.5 (L型)基因在植物受伤3 h后表达[47]。LecRK-Ⅰ.9在正常情况下高表达,植株受到损伤时,会触发细胞破裂并释放ATP,而LecRK-Ⅰ.9能识别损伤响应信号[67–68]。黑杨(Populus nigra)幼叶受伤后,PnLPK基因转录水平提高[12]。烟草LecRK1基因和水稻OsLecRK1基因在植株受到机械损伤胁迫后表达量上调,豌豆中PsLecRLK (L型)基因和辣椒(Capsicum annuum) CaLecRK-S.5基因参与机械损伤响应[69]。因此,凝集素类受体激酶参与机械损伤应答。
4.2 参与植物免疫已有研究证实,凝集素类受体激酶是植物-病原体作用中的PRR,对病原体的识别较为敏感(表 4)。除了磷酸化之外,泛素化和棕榈酰化也在维持植物细胞稳态、激活免疫受体复合物方面发挥着重要作用,如E3泛素连接酶OsPIE3能够改变水稻凝集素类受体激酶PID2的亚细胞定位,使其从质膜募集至核,降低稻瘟病抗性[75];植物免疫受体LecRK-Ⅰ.9能与拟南芥PAT5/9相互作用并磷酸化PAT5/9蛋白DHHC结构域,激活PAT5/9的棕榈酰化活性,激活PAT5/9棕榈酰化LecRK-Ⅰ.9,动态影响LecRK-Ⅰ.9受体蛋白的自我磷酸化和蛋白质降解[76]。
| 表 4 参与植物免疫的凝集素类受体激酶基因 Table 4 LecRLKs involved in plant immunity |
L型凝集素类受体激酶,参与植物的抗病过程。拟南芥[71]、黄瓜[77]、小麦[54]、大麦(Hordeum vulgare)[78]、辣椒[72]、苹果(Malus domestica)[79]的抗病性研究中,均鉴定出L型凝集素类受体激酶。拟南芥LecRK-Ⅰ.9参与调节茉莉酸(jasmonic acid, JA)信号通路,缺陷突变体在细胞壁防御和JA信号传导方面受到损害,对丁香假单胞菌、卵菌病原体、甘蓝疫霉和辣椒疫霉的敏感性增加,过表达LecRK-I.9基因增强了植株对病原体的抵抗力[80]。LecRK-Ⅰ.9与致病疫霉菌的效应蛋白互作,影响细胞壁与细胞膜的粘附来调节植物抗病力[81]。LecRK-I.5与LecRK-I.9可能存在协同效应,其双突变植株,加剧植株感染丁香假单胞菌[82]。
拟南芥LecRK-Ⅵ.2与类受体激酶BAK1互作组成受体复合物,通过细胞外烟酰胺腺嘌呤二核苷酸(extracellular nicotinamide adenine dinucleotide, eNAD+)信号触发系统获得抗性,同时LecRK-Ⅵ.2也是细胞外烟酰胺腺嘌呤二核苷酸磷酸(extracellular nicotinamide adenine dinucleotide, eNADP+)的潜在受体[83]。此外,LecRK-Ⅰ.8是eNAD+诱导的防御信号通路的组成部分,LecRK-Ⅰ.8作为昆虫卵触发的上游调节因子参与昆虫卵的识别[84]。LecRK-I.1与LecRK-I.8基因同源,LecRK-I.1参与昆虫卵诱导后的细胞死亡调控,这2个基因均参与SA介导的免疫相关的信号通路[81]。
研究表明,拟南芥的LecRK-V.2、LecRK-VII.1和LecRK-V.5基因,在针对细菌病原体的气孔介导的免疫反应中发挥作用,LecRK-Ⅴ.2和LecRK-Ⅶ.1参与气孔关闭[85];LecRK-Ⅴ.5通过对ABA应答调节气孔开闭[13]。病原体激活LecRK-IX.2转录, lecrk-IX.1和lecrk-IX.2突变体对疫霉菌的抗性受到损害,过表达植株抗病性增强,过表达LecRK-IX.2导致ROS触发的SA增加,SA升高时可导致LecRK-Ⅸ.2介导的细胞死亡,LecRK-Ⅸ.2可招募钙依赖性蛋白激酶,诱导RbohD磷酸化,触发拟南芥中ROS的产生,适量的ROS积累增强植物的抵抗力[86]。拟南芥AtLPK1基因过表达植株提高了对灰霉菌的抵抗力[58]。
在农作物中,L型凝集素类受体激酶的抗病功能也有研究。在小麦中,接种小麦白粉病病原体的植株,L型LecRK-V迅速上调,LecRK-V的过表达小麦植株,产生广谱白粉病抗性[53]。大麦Rphq2和Rph22基因,对非适应性叶锈病菌的抗性效果,比对适应性叶锈病菌的抗性效果更强,原因是适应性叶锈病菌通过降低配体识别,降低宿主受体的感知能力[78]。Woo等[72]报道辣椒CaLecRK-S.5基因对白粉病具有广谱抗性。黄瓜CsLecRK6.1基因受疫霉菌诱导表达[74, 77]。苹果MdLecRK-S.4.3基因在感染Valsa mali和Valsa pyri时,表现出病原体抗性,类受体胞质激酶基因PbePUB36过表达,干扰MdLecRK-S.4.3引起的免疫反应,MdLecRK-S.4.3和PbePUB36和/或MdBAK1相互作用,形成复杂的信号传导网络,介导宿主细胞的免疫应答[79]。
G型凝集素受体激酶也参与水稻、拟南芥、烟草和小麦的抗病过程,目前已报道参与植物抗病的G型凝集素类受体激酶主要有13个。水稻凝集素类受体激酶PID2参与抗稻瘟病菌,与E3泛素连接酶OsPUB15相互作用,磷酸化OsPUB15,调节植物细胞死亡和先天免疫,进一步研究表明PID2可与OsPUB15同源物OsPIE3相互作用,OsPIE3促进其在泛素-蛋白酶体系中的降解,从而破坏PID2的稳定性,并负调控水稻的抗稻瘟病性[75];水稻OslecRK不仅影响种子萌发,还有助于植物先天免疫[39]。水稻SDS2通过磷酸化E3连接酶SPL11来调控细胞程序性死亡和免疫[87];水稻PWL1负调节水稻对单黄胞菌的抗性,其突变体通过ROS、SA和JA的积累增强对细菌病原体的抗性[42]。
拟南芥LORE (G型)识别革兰氏阴性细菌中的中链3-羟基脂肪酸(3-OH-C10:0),3-OH-C10:0磷酸化LORE酪氨酸位点Y600,激活植物免疫[88]。研究表明,RDA2凝集素类受体激酶是DFPM ([5-(3, 4-dichlorophenyl)furan-2-yl]-piperidine-1-ylmethanethi-one)介导的免疫信号激活所必需的[89]。G型凝集素类受体激酶SBP1/2与类受体激酶SOBIR1、BAK1和RLP23互作,并调控多肽nlp20诱导的ROS、MAPK激活和防卫基因表达等免疫反应,SBP1和SBP2双突变体表现出对微生物模式分子诱导的防御基因表达和抗病力的缺陷[90]。ERN1是一个典型的免疫负调节因子,突变体表型为对根结线虫抗性增强[17]。
烟草NbLRK1通过激酶结构域,与致病疫霉菌INF1诱导素相互作用,病毒诱导的NbLRK1基因沉默,延迟本塞姆氏烟草中INF1介导的超敏反应[91];Nt-Sd-RLK在免疫信号转导途径中发挥作用[92]。据报道,本塞姆氏烟草的NbERK1 (G型)可调节辣椒疫霉的质外体效应子的感知,正向调节植物抗性[93];NbERK1与几丁质受体NbCERK1和NbLYK4形成复合物,正向调节几丁质信号和对核盘菌的抗性[94]。王建锋等[95]用小麦和条锈菌互作筛选出的TaLecRLK1基因(G型),在条锈菌侵染早期被诱导表达,表明其在小麦抗叶锈病中起正调控的作用。
4.3 植物-微生物共生互作凝集素类受体激酶家族参与植物-微生物共生(表 5)。根瘤菌细胞表面衍生的多糖,能被L型LecRLKs所识别,表明LecRLKs蛋白在豆科植物与根瘤菌的共生关系中,发挥配体识别的作用。蒺藜苜蓿的MtLecRK1;1 (G型)在氮缺乏条件下表达水平上调,过表达植株形成更多根瘤[14]。毛果杨PtLecRLK1 (G型)基因在拟南芥中过表达,可以将非寄主植物转化为菌根真菌(Laccaria bicolor)的宿主,在植物细胞之间建立菌丝网络,提高植物的适应能力[18–19]。酵母双杂交实验证明,豆科植物紫云英(Astragalus sinicus)的AsNIP43 (G型)与根瘤菌分泌的效应蛋白NopP互作,正向调节根瘤菌的结瘤数量[4]。
| 表 5 参与植物-真菌共生的凝集素类受体激酶基因 Table 5 LecRLKs genes involved in plant-fungus symbiotic interactions |
由于植物凝集素类受体蛋白激酶功能的多样性,针对LecRLKs的研究已经在植物抗性生物学领域取得显著进展。基于现有结果提出凝集素类受体激酶研究中存在的不足之处。
植物LecRLKs的起源、演化历史的研究极其缺乏,表现在多数植物的LecRLKs家族缺乏全基因组鉴定,目前仅在17种植物中得到鉴别,水稻中含有173个LecRLKs,明确功能的LecLRKs仅有14个,其他159个LecRLKs的功能尚未明确。不同物种LecRLKs家族的拷贝数目差别较大,表明全基因组复制及基因组进化速率,对LecRLKs家族的扩增/收缩进程影响较大,限于LecRLKs家族的比较基因组学研究的缺乏,LecRLKs在植物物种中的多样性、演化历史尚不明晰。通过比较不同物种中LecRLKs的进化速率,探索高度保守的结构域序列的生物意义,揭示植物LecRLKs抗逆高效优异性状形成的遗传基础和分子调控机制,有助于从细胞膜层面,理解植物生存策略。因此,LecRLKs家族的起源和演化历史研究对于揭示植物的生态适应性是至关重要的。
LecRLKs基因家族的信号传导途径及其潜在配体亟待开展。迄今为止,仅鉴定了LecRLKs的4种配体(eATP、eNAD+、eNADP+和3-OH-C10:0)[96],其他配体知之甚少。目前已知LecRK-Ⅰ.9和LecRK-Ⅰ.5可以结合ATP,通过磷酸化下游蛋白从而导致免疫反应的激活[82];LecRK-Ⅰ.8可识别结合NAD+, LecRK-I.8的突变抑制NAD+诱导的免疫反应, LecRK-Ⅵ.2是NAD+和NADP+的潜在受体,它通过与BAK1形成受体复合物感知NAD+,触发系统获得性抗性[83];LORE可识别结合3-OH-C10:0,介导拟南芥对假单胞菌和黄单胞菌的免疫反应,研究表明胞内3个受体激酶PBL34、PBL35、PBL36均能被LORE磷酸化,参与下游信号传递[88, 97]。对凝集素类受体蛋白的功能研究,主要基于表达量的差别, 缺乏其他实验证据。LecRLKs信号传导途径涉及细胞外寡糖信号的转导,后续的研究可使用气相色谱-质谱和核磁共振等分析方法,研究三维多糖结构; 多糖微阵列用于高效筛选蛋白质-多糖结合,建立LecRLKs的配体图谱;免疫共沉淀、蛋白质质谱分析方法,能有效筛选与凝集素类受体激酶互作的蛋白,进一步阐明凝集素类受体激酶的信号通路和调控机制。明确LecRLKs的配体和信号传导途径,阐明信号通路协调植物抗性生物学过程,解析该基因家族的亚功能化过程,对基于全基因组的高密度分子标记进行选择育种具有指导意义。
综上所述,凝集素类受体激酶研究在作物抗逆高效育种领域有广阔的应用前景。采用CRISPR-Cas9等基因编辑技术,精确调控LecRLKs表达量,培育抗逆高效作物新品种,Wang等[98]通过敲除L型凝集素类受体激酶基因OsCORK1,提高水稻对铜胁迫的耐受性。LecRLKs与昆虫或微生物之间的互作研究,挖掘具有重要育种价值的优异候选基因,以及它们的生态系统响应,有助于理解作物在自然生态系统中的生存策略。2050年地球人口将达到97亿,常规育种不能满足耕地面积减少及日益恶劣的生态环境,加速抗性基因家族的定向育种, 为作物抗虫基因工程提供新的候选基因。总之,凝集素类受体激酶的全基因组鉴定及逆境响应研究, 将为抗逆高效作物新品种的培育提供基因资源和理论基础。
| [1] |
WALKER J C. Structure and function of the receptor-like protein kinases of higher plants[J]. Plant Mol Biol, 1994, 26(5): 1599-1609. DOI:10.1007/BF00016492 |
| [2] |
SHUMAYLA, TYAGI S, UPADHYAY S K. Receptor-like kinases and environmental stress in plants[M]//SINGH S P, UPADHYAY S K, PANDEY A, et al. Molecular Approaches in Plant Biology and Environmental Challenges. Singapore: Springer, 2019: 79–102. doi: 10.1007/978-981-15-0690-1_4.
|
| [3] |
SUN J Q, JIANG J. Research progress on plant M/MLD-RLKs and their biological functions[J]. Acta Bot Boreali-Occid Sin, 2022, 42(5): 892-900. 孙嘉琪, 姜晶. 植物M/MLD类受体蛋白激酶及其生物学功能的研究进展[J]. 西北植物学报, 2022, 42(5): 892-900. DOI:10.7606/j.issn.1000-4025.2022.05.0892 |
| [4] |
LIU Y, LIN Y, WEI F, et al. G-type receptor-like kinase AsNIP43 interacts with rhizobia effector nodulation outer protein P and is required for symbiosis[J]. Plant Physiol, 2023, 193(2): 1527-1546. DOI:10.1093/plphys/kiad318 |
| [5] |
VAID N, PANDEY P K, TUTEJA N. Genome-wide analysis of lectin receptor-like kinase family from Arabidopsis and rice[J]. Plant Mol Biol, 2012, 80(4/5): 365-388. DOI:10.1007/s11103-012-9952-8 |
| [6] |
VAID N, MACOVEI A, TUTEJA N. Knights in action: Lectin receptor-like kinases in plant development and stress responses[J]. Mol Plant, 2013, 6(5): 1405-1418. DOI:10.1093/mp/sst033 |
| [7] |
PENG X Q, ZOU Y Q, LUO S W, et al. Research progress on lectin receptor-like kinases and their roles in mediation of plant disease resistance[J]. Plant Sci J, 2022, 40(1): 105-114. 彭小群, 邹雅琦, 骆素微, 等. 植物凝集素类受体激酶参与抗病的研究进展[J]. 植物科学学报, 2022, 40(1): 105-114. DOI:10.11913/PSJ.2095-0837.2022.10105 |
| [8] |
WANG Y, WEIDE R, GOVERS F, et al. L-type lectin receptor kinases in Nicotiana benthamiana and tomato and their role in Phytophthora resistance[J]. J Exp Bot, 2015, 66(21): 6731-6743. DOI:10.1093/jxb/erv379 |
| [9] |
YANG Y, LABBÉ J, MUCHERO W, et al. Genome-wide analysis of lectin receptor-like kinases in Populus[J]. BMC Genom, 2016, 17(1): 699. DOI:10.1186/S12864-016-3026-2 |
| [10] |
LI C H, WANG G, ZHAO J L, et al. The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice[J]. Plant Cell, 2014, 26(6): 2538-2553. DOI:10.1105/tpc.114.125187 |
| [11] |
HERVÉ C, SERRES J, DABOS P, et al. Characterization of the Arabidopsis lecRK-a genes: Members of a superfamily encoding putative receptors with an extracellular domain homologous to legume lectins[J]. Plant Mol Biol, 1999, 39(4): 671-682. DOI:10.1023/A:1006136701595 |
| [12] |
NISHIGUCHI M, YOSHIDA K, SUMIZONO T, et al. A receptor-like protein kinase with a lectin-like domain from lombardy poplar: Gene expression in response to wounding and characterization of phosphorylation activity[J]. Mol Gen Genomics, 2002, 267(4): 506-514. DOI:10.1007/s00438-002-0683-4 |
| [13] |
WANG M L, LUO S W, LI X S, et al. Research advances on plant lectin receptor-like kinases in abiotic stress response[J]. Guihaia, 2023, 43(11): 2159-2169. 王梦龙, 骆素微, 李晓诗, 等. 植物凝集素类受体激酶参与非生物胁迫响应的研究进展[J]. 广西植物, 2023, 43(11): 2159-2169. DOI:10.11931/guihaia.gxzw202302002 |
| [14] |
NAVARRO-GOCHICOA M T, CAMUT S, TIMMERS A C J, et al. Characterization of four lectin-like receptor kinases expressed in roots of Medicago truncatula structure, location, regulation of expression, and potential role in the symbiosis with Sinorhizobium meliloti[J]. Plant Physiol, 2003, 133(4): 1893-1910. DOI:10.1104/pp.103.027680 |
| [15] |
SUN Y L, QIAO Z Z, MUCHERO W, et al. Lectin receptor-like kinases: The sensor and mediator at the plant cell surface[J]. Front Plant Sci, 2020, 11: 596301. DOI:10.3389/fpls.2020.596301 |
| [16] |
KAUR A, SHARMA A, MADHU, et al. Analysis of lectin receptor-like kinases and their functions in higher plants[M]//UPADHYAY S K, SHUMAYLA. Plant Receptor-Like Kinases. America: Academic Press, 2023: 167–182. doi: 10.1016/B978-0-323-90594-7.00008-9.
|
| [17] |
ZHOU D M, GODINEZ-VIDAL D, HE J M, et al. A G-type lectin receptor kinase negatively regulates Arabidopsis immunity against root-knot nematodes[J]. Plant Physiol, 2023, 193(1): 721-735. DOI:10.1093/plphys/kiad253 |
| [18] |
LABBÉ J, MUCHERO W, CZARNECKI O, et al. Mediation of plantmycorrhizal interaction by a lectin receptor-like kinase[J]. Nat Plants, 2019, 5(7): 676-680. DOI:10.1038/S41477-019-0469-X |
| [19] |
SHRESTHA H, YAO T, QIAO Z Z, et al. Lectin receptor-like kinase signaling during engineered ectomycorrhiza colonization[J]. Cells, 2023, 12(7): 1082. DOI:10.3390/cells12071082 |
| [20] |
LIU P L, HUANG Y, SHI P H, et al. Duplication and diversification of lectin receptor-like kinases (LecRLK) genes in soybean[J]. Sci Rep, 2018, 8(1): 5861. DOI:10.1038/S41598-018-24266-6 |
| [21] |
WALKER J C, ZHANG R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica[J]. Nature, 1990, 345(6277): 743-746. DOI:10.1038/345743a0 |
| [22] |
HERVÉ C, DABOS P, GALAUD J P, et al. Characterization of an Arabidopsis thaliana gene that defines a new class of putative plant receptor kinases with an extracellular lectin-like domain[J]. J Mol Biol, 1996, 258(5): 778-788. DOI:10.1006/jmbi.1996.0286 |
| [23] |
SHUMAYLA, SHARMA S, PANDEY A K, et al. Molecular characterization and global expression analysis of lectin receptor kinases in bread wheat (Triticum aestivum)[J]. PLoS One, 2016, 11(4): e0153925. DOI:10.1371/journal.pone.0153925 |
| [24] |
ZHAO W, LIU Y W, ZHOU J M, et al. Genome-wide analysis of the lectin receptor-like kinase family in foxtail millet (Setaria italica L.)[J]. Plant Cell Tiss Organ Cult, 2016, 127(2): 335-346. DOI:10.1007/s11240-016-1053-y |
| [25] |
ZHAO T M, WANG J Y, ZHANG B L, et al. Genome-wide analysis of lectin receptor-like kinases in tomato (Solanum lycopersicum) and its association with the infection of tomato yellow leaf curl virus[J]. Plant Mol Biol Rep, 2018, 36(3): 429-438. DOI:10.1007/s11105-018-1091-1 |
| [26] |
MA N, LIU C X, LI H, et al. Genome-wide identification of lectin receptor kinases in pear: Functional characterization of the L-type LecRLK gene PbLRK138[J]. Gene, 2018, 661: 11-21. DOI:10.1016/j.gene.2018.03.077 |
| [27] |
GUO J B, DUAN H, XUAN L, et al. Identification and functional analysis of LecRLK genes in Taxodium 'Zhongshanshan'[J]. PeerJ, 2019, 7: e7498. DOI:10.7717/peerj.7498 |
| [28] |
ZHANG W N, CHEN Z J, KANG Y C, et al. Genome-wide analysis of lectin receptor-like kinases family from potato (Solanum tuberosum L.)[J]. PeerJ, 2020, 8: e9310. DOI:10.7717/peerj.9310 |
| [29] |
HAN H Y, MU X P, WANG P F, et al. Identification of LecRLK gene family in Cerasus humilis through genomic-transcriptomic data mining and expression analyses[J]. PLoS One, 2021, 16(7): e0254535. DOI:10.1371/journal.pone.0254535 |
| [30] |
WANG Z T, REN H, XU F, et al. Genome-wide characterization of lectin receptor kinases in Saccharum spontaneum L. and their responses to Stagonospora tainanensis infection[J]. Plants, 2021, 10(2): 322. DOI:10.3390/plants10020322 |
| [31] |
SINGH P, MISHRA A K, SINGH C M. Genome-wide identification and characterization of Lectin receptor-like kinase (LecRLK) genes in mungbean (Vigna radiata L. Wilczek)[J]. J Appl Genetics, 2021, 62(2): 223-234. DOI:10.1007/s13353-021-00613-8 |
| [32] |
HAIDER M S, DE BRITTO S, NAGARAJ G, et al. Genome-wide identification, diversification, and expression analysis of lectin receptor-like kinase (LecRLK) gene family in cucumber under biotic stress[J]. Int J Mol Sci, 2021, 22(12): 6585. DOI:10.3390/ijms22126585 |
| [33] |
SUN Y, ZHAO X H, GAO Y H, et al. Genome-wide analysis of lectin receptor-like kinases (LecRLKs) in sweet cherry (Prunus avium) and reveals PaLectinL16 enhances sweet cherry resistance with salt stress[J]. Environ Exp Bot, 2022, 194: 104751. DOI:10.1016/j.envexpbot.2021.104751 |
| [34] |
HE Z Y, ZOU T, XIAO Q, et al. An L-type lectin receptor-like kinase promotes starch accumulation during rice pollen maturation[J]. Development, 2021, 148(6): dev196378. DOI:10.1242/dev.196378 |
| [35] |
PENG X Q, WANG M L, LI Y Q, et al. Lectin receptor kinase OsLecRK-S.7 is required for pollen development and male fertility[J]. J Integr Plant Biol, 2020, 62(8): 1227-1245. DOI:10.1111/jipb.12897 |
| [36] |
WANG B, FANG R Q, ZHANG J, et al. Rice LecRK5 phosphorylates a UGPase to regulate callose biosynthesis during pollen development[J]. J Exp Bot, 2020, 71(14): 4033-4041. DOI:10.1093/jxb/eraa180 |
| [37] |
ZHANG X, ZHAO G C, TAN Q, et al. Rice pollen aperture formation is regulated by the interplay between OsINP1 and OsDAF1[J]. Nat Plants, 2020, 6(4): 394-403. DOI:10.1038/s41477-020-0630-6 |
| [38] |
BI Z Z. Functional analysis of Osl-LecRK7 in rice[D]. Xinxiang: Henan Normal University, 2013. 毕真真. 水稻OsL-LecRK7基因的功能研究[D]. 新乡: 河南师范大学, 2013. |
| [39] |
CHENG X Y, WU Y, GUO J P, et al. A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination[J]. Plant J, 2013, 76(4): 687-698. DOI:10.1111/tpj.12328 |
| [40] |
LI B, LI Y X, QIU M D, et al. OsSRK1, a lectin receptor-like kinase, controls plant height by mediating internode elongation in Oryza sativa L.[J]. Mol Breed, 2022, 42(12): 74. DOI:10.1007/s11032-022-01340-6 |
| [41] |
CHEN L J, WURIYANGHAN H, ZHANG Y Q, et al. An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark-induced leaf senescence in rice[J]. Plant Physiol, 2013, 163(4): 1752-1765. DOI:10.1104/pp.113.224881 |
| [42] |
XU J M, WANG C L, WANG F J, et al. PWL1, a G-type lectin receptor-like kinase, positively regulates leaf senescence and heat tolerance but negatively regulates resistance to Xanthomonas oryzae in rice[J]. Plant Biotechnol J, 2023, 21(12): 2525-2545. DOI:10.1111/pbi.14150 |
| [43] |
WAN J R, PATEL A, MATHIEU M, et al. A lectin receptor-like kinase is required for pollen development in Arabidopsis[J]. Plant Mol Biol, 2008, 67(5): 469-482. DOI:10.1007/s11103-008-9332-6 |
| [44] |
XIN Z Y, WANG A Y, YANG G H, et al. The Arabidopsis A4 subfamily of lectin receptor kinases negatively regulates abscisic acid response in seed germination[J]. Plant Physiol, 2009, 149(1): 434-444. DOI:10.1104/pp.108.130583 |
| [45] |
XIAO W J, HU S, ZOU X X, et al. Lectin receptor-like kinase LecRK-Ⅷ.2 is a missing link in MAPK signaling-mediated yield control[J]. Plant Physiol, 2021, 187(1): 303-320. DOI:10.1093/plphys/kiab241 |
| [46] |
LIN X X. Research of LecRK-VIII. 2-mediated involved in hypocotyl growth and flowering time in Arabidopsis [D]. Changsha: Hunan University, 2020. 林晓霞. LecRK-VIII. 2基因调控拟南芥下胚轴生长及开花时间的研究[D]. 长沙: 湖南大学, 2020. |
| [47] |
RIOU C, HERVÉ C, PACQUIT V, et al. Expression of an Arabidopsis lectin kinase receptor gene, lecRK-a1, is induced during senescence, wounding and in response to oligogalacturonic acids[J]. Plant Physiol Biochem, 2002, 40(5): 431-438. DOI:10.1016/S0981-9428(02)01390-6 |
| [48] |
MICOL-PONCE R, GARCÍA-ALCÁZAR M, LEBRÓN R, et al. Tomato POLLEN DEFICIENT 2 encodes a G-type lectin receptor kinase required for viable pollen grain formation[J]. J Exp Bot, 2023, 74(1): 178-193. DOI:10.1093/jxb/erac419 |
| [49] |
HUANG R L. Functional analysis of AhLecRK9 and AhLEAs in peanut under aluminum stress[D]. Nanning: Guangxi University, 2022. 黄若兰. 花生AhLecRK9和AhLEAs在铝胁迫下的功能研究[D]. 南宁: 广西大学, 2022. |
| [50] |
SAIDOU M, ZHANG Z Y. The L-type lectin-like receptor kinase gene TaLecRK-IV.1 regulates the plant height in wheat[J]. Int J Mol Sci, 2022, 23(15): 8208. DOI:10.3390/ijms23158208 |
| [51] |
ZUO K J, ZHAO J Y, WANG J, et al. Molecular cloning and characterization of GhlecRK, a novel kinase gene with lectin-like domain from Gossypium hirsutum[J]. DNA Seq, 2004, 15(1): 58-65. DOI:10.1080/1042517042000191454 |
| [52] |
ZHANG C, GUO X H, XIE H L, et al. Quantitative phosphoproteomics of lectin receptor‐like kinase Ⅵ.4 dependent abscisic acid response in Arabidopsis thaliana[J]. Physiol Plant, 2019, 165(4): 728-745. DOI:10.1111/ppl.12763 |
| [53] |
LV D, WANG G, XIONG L R, et al. Genome-wide identification and characterization of lectin receptor-like kinase gene family in cucumber and expression profiling analysis under different treatments[J]. Genes, 2020, 11(9): 1032. DOI:10.3390/genes11091032 |
| [54] |
WANG Z K, CHENG J Y, FAN A Q, et al. LecRK-V, an L-type lectin receptor kinase in Haynaldia villosa, plays positive role in resistance to wheat powdery mildew[J]. Plant Biotechnol J, 2018, 16(1): 50-62. DOI:10.1111/pbi.12748 |
| [55] |
JIAO M C, CAI L G, WEI J, et al. Research progress on the physiological harms of salt stress and the adaptation mechanism of plants[J]. J Changchun Norm Univ, 2023, 42(6): 125-132. 焦明翠, 蔡立格, 魏健, 等. 盐胁迫的生理危害与植物的适应机制研究进展[J]. 长春师范大学学报, 2023, 42(6): 125-132. DOI:10.3969/j.issn.1008-178X.2023.06.018 |
| [56] |
HE X J, ZHANG Z G, YAN D Q, et al. A salt-responsive receptor-like kinase gene regulated by the ethylene signaling pathway encodes a plasma membrane serine/threonine kinase[J]. Theor Appl Genet, 2004, 109(2): 377-383. DOI:10.1007/s00122-004-1641-9 |
| [57] |
DENG K Q, WANG Q M, ZENG J X, et al. A lectin receptor kinase positively regulates ABA response during seed germination and is involved in salt and osmotic stress response[J]. J Plant Biol, 2009, 52(6): 493-500. DOI:10.1007/s12374-009-9063-5 |
| [58] |
HUANG P, JU H W, MIN J H, et al. Overexpression of L-type lectin-like protein kinase 1 confers pathogen resistance and regulates salinity response in Arabidopsis thaliana[J]. Plant Sci, 2013, 203-204: 98-106. DOI:10.1016/j.plantsci.2012.12.019 |
| [59] |
LI M L. Preliminary study on the function of LecRKIII. 2 gene in Arabidopsis thaliana[D]. Changsha: Hunan University, 2019. 李美玲. 拟南芥LecRKIII. 2基因的功能初步研究[D]. 长沙: 湖南大学, 2019. doi: 10.27135/d.cnki.ghudu.2019.002852. |
| [60] |
SUN M Z, QIAN X, CHEN C, et al. Ectopic expression of GsSRK in Medicago sativa reveals its involvement in plant architecture and salt stress responses[J]. Front Plant Sci, 2018, 9: 226. DOI:10.3389/fpls.2018.00226 |
| [61] |
ZHANG Y Z, FANG Q W, ZHENG J Q, et al. GmLecRlk, a lectin receptor-like protein kinase, contributes to salt stress tolerance by regulating salt-responsive genes in soybean[J]. Int J Mol Sci, 2022, 23(3): 1030. DOI:10.3390/ijms23031030 |
| [62] |
JOSHI A, DANG H Q, VAID N, et al. Pea lectin receptor-like kinase promotes high salinity stress tolerance in bacteria and expresses in response to stress in planta[J]. Glycoconj J, 2010, 27(1): 133-150. DOI:10.1007/s10719-009-9265-6 |
| [63] |
MA N, WANG J Y, KAN J L, et al. Functional analysis of a salt-induced lectin receptor-like kinase PcLRLK066 of pear[J]. Jiangsu J Agric Sci, 2017, 33(2): 404-411. 马娜, 王金彦, 阚家亮, 等. 一个响应盐胁迫的梨凝集素受体蛋白激酶PcLRLK066的功能分析[J]. 江苏农业学报, 2017, 33(2): 404-411. DOI:10.3969/j.issn.1000-4440.2017.02.026 |
| [64] |
WU F L, QU D H, ZHANG X, et al. PaLectinL7 enhances salt tole-rance of sweet cherry by regulating lignin deposition in connection with PaCAD1[J]. Tree Physiol, 2023, 43(11): 1986-2000. DOI:10.1093/treephys/tpad099 |
| [65] |
LU X T. Mechanism of action of lectin receptor like kinase LecRKIII. 1 and LecRKIII. 2 genes in Arabidopsis thaliana [D]. Changsha: Hunan University, 2016. 陆秀涛. 拟南芥凝集素类受体激酶基因LecRKIII. 1和LecRKIII. 2的作用机制研究[D]. 长沙: 湖南大学, 2016. |
| [66] |
LIU S H, WANG J, CHEN K S, et al. The L-type lectin receptor-like kinase (PnLecRLK1) from the Antarctic moss Pohlia nutans enhances chilling-stress tolerance and abscisic acid sensitivity in Arabidopsis[J]. Plant Growth Regul, 2017, 81(3): 409-418. DOI:10.1007/s10725-016-0217-4 |
| [67] |
CHOI J, TANAKA K, CAO Y R, et al. Identification of a plant receptor for extracellular ATP[J]. Science, 2014, 343(6168): 290-294. DOI:10.1126/science.343.6168.290 |
| [68] |
TANAKA K, CHOI J, CAO Y R, et al. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants[J]. Front Plant Sci, 2014, 5: 446. DOI:10.3389/fpls.2014.00446 |
| [69] |
VAID N, PANDEY P, SRIVASTAVA V K, et al. Pea lectin receptor-like kinase functions in salinity adaptation without yield penalty, by alleviating osmotic and ionic stresses and upregulating stress-responsive genes[J]. Plant Mol Biol, 2015, 88(1/2): 193-206. DOI:10.1007/s11103-015-0319-9 |
| [70] |
CUI X X. Role analysis of the lectin receptor-like kinase gene OsLecRK1 in stress resistance of rice[D]. Xiamen: Xiamen University, 2012. 崔欣欣. 凝集素类受体蛋白激酶基因OsLecRK1在水稻抗胁迫反应中作用的解析[D]. 厦门: 厦门大学, 2012. |
| [71] |
DESCLOS-THEVENIAU M, ARNAUD D, HUANG T Y, et al. The Arabidopsis lectin receptor kinase LecRK-Ⅴ.5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000[J]. PLoS Pathog, 2012, 8(2): e1002513. DOI:10.1371/journal.ppat.1002513 |
| [72] |
WOO J Y, JEONG K J, KIM Y J, et al. CaLecRK-S.5, a pepper L-type lectin receptor kinase gene, confers broad-spectrum resistance by activating priming[J]. J Exp Bot, 2016, 67(19): 5725-5741. DOI:10.1093/jxb/erw336 |
| [73] |
SUN X L, YU Q Y, TANG L L, et al. GsSRK, a G-type lectin S-receptor-like serine/threonine protein kinase, is a positive regulator of plant tolerance to salt stress[J]. J Plant Physiol, 2013, 170(5): 505-515. DOI:10.1016/j.jplph.2012.11.017 |
| [74] |
GILARDONI P A, HETTENHAUSEN C, BALDWIN I T, et al. Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insectmediated inhibition of induced defense responses during Manduca sexta herbivory[J]. Plant Cell, 2011, 23(9): 3512-3532. DOI:10.1105/tpc.111.088229 |
| [75] |
WANG K, LI S, CHEN L X, et al. E3 ubiquitin ligase OsPIE3 desta-bilises the B-lectin receptor-like kinase PID2 to control blast disease resistance in rice[J]. New Phytol, 2023, 237(5): 1826-1842. DOI:10.1111/nph.18637 |
| [76] |
CHEN D Q, HAO F S, MU H Q, et al. S-acylation of P2K1 mediates extracellular ATP-induced immune signaling in Arabidopsis[J]. Nat Commun, 2021, 12(1): 2750. DOI:10.1038/s41467-021-22854-1 |
| [77] |
WU T Q, WANG R, XU X M, et al. Cucumis sativus L-type lectin receptor kinase (CsLecRK) gene family response to Phytophthora melonis, Phytophthora capsici and water immersion in disease resistant and susceptible cucumber cultivars[J]. Gene, 2014, 549(2): 214-222. DOI:10.1016/j.gene.2014.07.058 |
| [78] |
WANG Y J, SUBEDI S, DE VRIES H, et al. Orthologous receptor kinases quantitatively affect the host status of barley to leaf rust fungi[J]. Nat Plants, 2019, 5(11): 1129-1135. DOI:10.1038/s41477-019-0545-2 |
| [79] |
SUN E, YU H Q, CHEN Z J, et al. Enhanced Valsa canker resistance conferred by expression of MdLecRK-S.4.3 in Pyrus betulifolia is largely suppressed by PbePUB36[J]. J Exp Bot, 2023, 74(14): 3998-4013. DOI:10.1093/jxb/erad126 |
| [80] |
BALAGUÉ C, GOUGET A, BOUCHEZ O, et al. The Arabidopsis thaliana lectin receptor kinase LecRK‐Ⅰ.9 is required for full resistance to Pseudomonas syringae and affects jasmonate signalling[J]. Mol Plant Pathol, 2017, 18(7): 937-948. DOI:10.1111/mpp.12457 |
| [81] |
GROUX R, STAHL E, GOUHIER-DARIMONT C, et al. Arabidopsis natural variation in insect egg-induced cell death reveals a role for LECTIN RECEPTOR KINASE-Ⅰ.1[J]. Plant Physiol, 2021, 185(1): 240-255. DOI:10.1093/plphys/kiaa022 |
| [82] |
PHAM A Q, CHO S H, NGUYEN C T, et al. Arabidopsis lectin receptor kinase P2K2 is a second plant receptor for extracellular ATP and contributes to innate immunity[J]. Plant Physiol, 2020, 183(3): 1364-1375. DOI:10.1104/pp.19.01265 |
| [83] |
WANG C G, HUANG X E, LI Q, et al. Extracellular pyridine nucleotides trigger plant systemic immunity through a lectin receptor kinase/BAK1 complex[J]. Nat Commun, 2019, 10(1): 4810. DOI:10.1038/s41467-019-12781-7 |
| [84] |
GOUHIER-DARIMONT C, STAHL E, GLAUSER G, et al. The Arabidopsis lectin receptor kinase LecRK-Ⅰ.8 is involved in insect egg perception[J]. Front Plant Sci, 2019, 10: 623. DOI:10.3389/fpls.2019.00623 |
| [85] |
YEKONDI S, LIANG F C, OKUMA E, et al. Nonredundant functions of Arabidopsis LecRK-Ⅴ.2 and LecRK-Ⅶ.1 in controlling stomatal immunity and jasmonate-mediated stomatal closure[J]. New Phytol, 2018, 218(1): 253-268. DOI:10.1111/nph.14953 |
| [86] |
LUO X M, XU N, HUANG J K, et al. A lectin receptor-like kinase mediates pattern-triggered salicylic acid signaling[J]. Plant Physiol, 2017, 174(4): 2501-2514. DOI:10.1104/pp.17.00404 |
| [87] |
FAN J B, BAI P F, NING Y S, et al. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice[J]. Cell Host Microbe, 2018, 23(4): 498-510.e5. DOI:10.1016/j.chom.2018.03.003 |
| [88] |
LUO X M, WU W, LIANG Y B, et al. Tyrosine phosphorylation of the lectin receptor-like kinase LORE regulates plant immunity[J]. EMBO J, 2020, 39(4): e102856. DOI:10.15252/embj.2019102856 |
| [89] |
PARK J, KIM T H, TAKAHASHI Y, et al. Chemical genetic identification of a lectin receptor kinase that transduces immune responses and interferes with abscisic acid signaling[J]. Plant J, 2019, 98(3): 492-510. DOI:10.1111/tpj.14232 |
| [90] |
BAO Y Z, LI Y X, CHANG Q, et al. A pair of G-type lectin receptor-like kinases modulates nlp20-mediated immune responses by coupling to the RLP23 receptor complex[J]. J Integr Plant Biol, 2023, 65(5): 1312-1327. DOI:10.1111/jipb.13449 |
| [91] |
KANZAKI H, SAITOH H, TAKAHASHI Y, et al. NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death[J]. Planta, 2008, 228(6): 977-987. DOI:10.1007/s00425-008-0797-y |
| [92] |
SANABRIA N M, VAN HEERDEN H, DUBERY I A. Molecular characterisation and regulation of a Nicotiana tabacum S-domain receptor-like kinase gene induced during an early rapid response to lipopolysaccharides[J]. Gene, 2012, 501(1): 39-48. DOI:10.1016/j.gene.2012.03.073 |
| [93] |
PI L, YIN Z Y, DUAN W W, et al. A G-type lectin receptor-like kinase regulates the perception of oomycete apoplastic expansin-like proteins[J]. J Integr Plant Biol, 2022, 64(1): 183-201. DOI:10.1111/jipb.13194 |
| [94] |
PI L, ZHANG Y F, WANG J H, et al. A G-type lectin receptor-like kinase in Nicotiana benthamiana enhances resistance to the fungal pathogen Sclerotinia sclerotiorum by complexing with CERK1/LYK4[J]. Phytopathol Res, 2023, 5(1): 27. DOI:10.1186/s42483-023-00182-0 |
| [95] |
WANG J F, GAN P F, XU J H, et al. Identification and functional analysis of TaLecRLK1 in wheat resistance to stripe rust[J]. Acta Phytopathol Res, 2023, 53(1): 61-71. 王建锋, 甘鹏飞, 徐静华, 等. 小麦凝集素类受体激酶TaLecRLK1的鉴定及抗锈病功能分析[J]. 植物病理学报, 2023, 53(1): 61-71. DOI:10.13926/j.cnki.apps.000840 |
| [96] |
LIU L, LIU J, XU N. Ligand recognition and signal transduction by lectin receptor-like kinases in plant immunity[J]. Front Plant Sci, 2023, 14: 1201805. |
| [97] |
SCHELLENBERGER R, CROUZET J, NICKZAD A, et al. Bacterial rhamnolipids and their 3-hydroxyalkanoate precursors activate Arabidopsis innate immunity through two independent mechanisms[J]. Proc Natl Acad Sci USA, 2021, 118(39): e2101366118. DOI:10.1073/pnas.2101366118 |
| [98] |
WANG K, LI S, YANG Z Y, et al. L-type lectin receptor-like kinase OsCORK1 as an important negative regulator confers copper stress tolerance in rice[J]. J Hazard Mat, 2023, 459: 132214. DOI:10.1016/j.jhazmat.2023.132214 |
 2025, Vol. 33
2025, Vol. 33


