2. 宁夏医科大学药学院, 银川 750004;
3. 山东省药学科学院, 山东省化学药物重点实验室, 济南 250101
2. College of Pharmacy, Ningxia Medical University, Yinchuan 750004, China;
3. Shandong Key Laboratory of Chemical Drugs, Shandong Academy of Pharmaceutical Sciences, Jinan 250101, China
光叶兔耳风(Ainsliaea glabra)是我国常用民间草药,为菊科(Compositae)多年生草本植物[1]。主治肺痨咯血,跌打损伤,具有养阴清肺,祛瘀止血的功效。清代赵学敏编著《本草纲目拾遗》为较早记载。地方药品标准如福建省和江西省《中药饮片炮制规范》,湖北省《中药材标准》均有收录。
兔耳风属植物在我国分布较多,约有50余种,常见于长江流域,云贵川,两江两广等省份均有分布[2]。该属植物化学成分的研究较少,早期研究表明,兔耳风属植物富含萜类,主要为倍半萜、单萜和三萜[3],其中杏香兔耳风(A. fragrans)、大头兔耳风(A. macrocephala)和云南兔耳风(A. yunnanensis)研究相对较多,而对光叶兔耳风(A. glabra)的化学成分鲜有报道。陈亚萍等[4–5]对光叶兔耳风的80%乙醇水溶液提取物进行了初步化学成分和药理活性研究,报道了28个化合物,其中3个为倍半萜类,1个ainsliaea acid A具有较为明显的抗炎活性[5]。为了进一步明确兔耳风的药效物质基础,推动民间中草药的开发和利用,本课题组对采集于四川峨眉山的光叶兔耳风90%乙醇提取物进行了系统的化学成分研究,从其石油醚萃取段分离得到了10个单体化合物,利用一维(1D)、二维(2D)核磁共振技术(nuclear magnetic resonance spectroscopy, NMR)、质谱、红外光谱以及与文献比对等方法确定其化学结构,并且对分离得到的单体化合物进行抗菌活性测试。
1 材料和方法 1.1 材料和仪器光叶兔耳风药材于2017年6月采自四川省峨眉山市,由四川省食品药品学校秦运潭副教授鉴定为光叶兔耳风(Ainsliaea glabra)地上全草。标本(No. AG201706-1)保存于北方民族大学植物标本室。金黄色葡萄球菌(ATCC8739)和白色念珠菌(ATCC 10231)均保存于北方民族大学化学与化学工程学院。
A Bruker Avance AVIII-600、500、400核磁共振仪(美国Bruker公司);Agilent 6530液相色谱质谱联用仪(美国安捷伦科技公司);高效液相色谱仪(美国Waters公司);zorbax XDB-C18色谱柱(250 mm× 10 mm, 5 μm; Agilent, American);正向硅胶(200~300目,青岛海洋化工厂);葡聚糖凝胶Sephadex LH-20 (瑞典Amersham Pharmacia公司);薄层硅胶板(GF254, 青岛海洋化工厂);分析纯试剂(天津大茂试剂厂); 色谱纯试剂(安徽天地高纯溶剂有限公司);胰蛋白胨(上海博微生物科技有限公司);酵母浸出膏(上海博微生物科技有限公司);琼脂粉(青岛高科园海博生物科技有限公司);葡萄糖(天津福晨化学试剂有限公司);盐酸去甲万古霉素(> 98%,华北制药集团新药开发有限责任公司);分析纯重铬酸钾(北京伊诺凯科技有限公司)。
1.2 提取分离取光叶兔儿风干燥全草(干重6.5 kg)粉碎,加90%乙醇回流提取3次,浓缩回收乙醇,得300 g浸膏。加水使其混悬,分别用石油醚、乙酸乙酯和正丁醇萃取,回收溶剂,减压蒸干得石油醚提取物74.9 g、乙酸乙酯提取物54.0 g和正丁醇提取物56.5 g。其中石油醚提取物74.9 g,经硅胶柱色谱(石油醚-乙酸乙酯,50:1~1:1)分离,得到6个主要流份P1~P6。P2部分反复经硅胶柱色谱, 最后经凝胶Sephadex LH-20柱色谱纯化得化合物8 (11 mg)、9 (20 mg)和10 (25 mg);P3部分经硅胶柱色谱(石油醚-乙酸乙酯,30:1)等度洗脱的3个主要流分P3-1~P3-3, P3-2经Sephadex LH-20柱色谱纯化得化合物1 (15 mg)、2 (8 mg)和3 (12 mg);P5洗脱液经Sephadex LH-20柱色谱分离得到3个主要流分P5-1~P5-3, 其中P5-2经高效液相色谱(水: 乙腈,40:60)分离得到化合物4 (7 mg)、5 (11 mg)、6 (12 mg)和7 (5 mg)。化合物1~10化学结构如图 1。
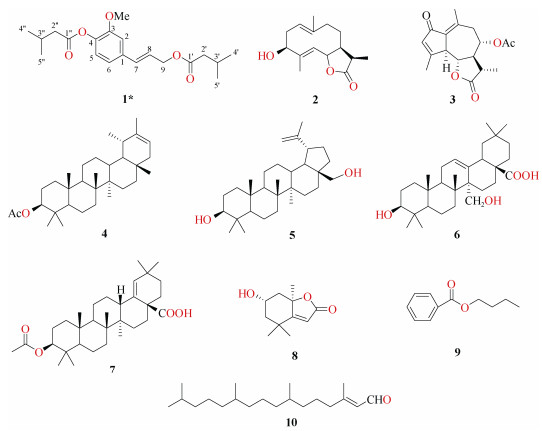
|
图 1 化合物1~10化学结构。*: 新结构化合物。 Fig. 1 Structures of compounds 1-10. *: New structural compounds. |
化合物1 无色油状物,UV254下有清晰暗斑,硫酸乙醇(5%)加热显紫色。高分辨质谱给出分子离子峰m/z 371.1822 [M + Na]+(计算值为371.193 7, C20H28O5Na+),结合13C核磁共振波谱数据推测其分子式为C20H28O5,Ω=7。红外光谱显示羰基(1 763.3 cm–1, 1 731.3 cm–1)、苯环(968 cm–1)特征吸收峰。1H NMR (CDCl3, 400 MHz)显示δppm ABX三取代的苯环氢信号6.98 (1H, d, J = 1.2 Hz, H-2)、6.95 (1H, dd, J = 6.8, 1.2 Hz, H-6)、6.96 (1H, d, J = 6.8, H-5)。一对反式双键质子信号:6.63 (1H, brd, J = 16.0, H-7)、6.23 (1H, dt, J = 16.0, 6.0 Hz, H-8);4个双峰甲基质子信号0.97 (6H, d, J = 6.4 Hz, 2×CH3)、1.07 (6H, d, J = 6.4 Hz, 2×CH3)。1个甲氧基信号3.83 (3H, s, OCH3);13C NMR (CDCl3, 100 MHz)显示2个酯羰基信号173.0 (s, CO)、171.2 (s, CO);1H-1H COSY实验(图 2)给出2个异丁基片段2×-CH2CH (CH3)2,1个烯丙基-CH2-CH=CH-片段。至此,确定该化合物有1个苯环、2个异丁基、1个烯丙基、1个甲氧基结构片段。HMBC实验有下列远程相关:H-7和C-2/C-6,H-9和C-1′,H-2′和C-1′,H-2″和C-1″,可以将上述片段连接起来。同时,HMBC实验给出-OMe与C-3相关,说明甲氧基连到苯环C-3位置上。至此,化合物1结构得以确定,并且在SciFinders数据库中进行搜索,没有检索到完全一致的结构,提示化合物1为新结构,命名为松柏烯醇二异戊酸酯(pinerenol diisovalerate)。
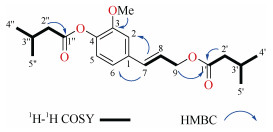
|
图 2 化合物1的1H-1H COSY和主要的HMBC相关 Fig. 2 1H-1H COSY and major HMBC correlations of compound 1 |
1H NMR (CDCl3, 400 MHz): δ 6.98 (1H, d, J = 1.2 Hz, H-2), 6.95 (1H, dd, J = 6.8, 1.2 Hz, H-6), 6.96 (1H, d, J = 6.8, H-5), 6.63 (1H, br d, J = 16.0, H-7), 6.23 (1H, dt, J = 16.0, 6.0 Hz, H-8), 4.72 (2H, dd, J = 5.2, 1.2 Hz, H2-9), 3.83 (3H, s, OCH3), 2.44 (2H, d, J = 6.4 Hz, H2-2″), 2.27 (1H, m, H-3″), 2.22 (2H, d, J = 6.4 Hz, H2-2′), 2.12 (1H, m, H-3′), 1.06 (6H, d, J = 6.8 Hz, H3-4″, H3-5″), 0.97 (6H, d, J = 6.8 Hz, H3-4′, H3-5′); 13C NMR (CDCl3, 100 MHz): δ 135.2 (s, C-1), 110.2 (d, C-2), 151.2 (s, C-3), 139.7 (s, C-4), 123.7 (d, C-5), 122.9 (d, C-6), 133.5 (d, C-7), 119.3 (d, C-8), 64.6 (t, C-9), 172.9 (s, C-1′), 171.0 (s, C-1″), 43.4 (t, C-2′), 43.0 (t, C-2″), 25.9 (d, C-3′), 25.7 (d, C-3″), 22.4 (q, C-4′, 5′), 22.3 (q, C-4″, 5″), 55.8 (q, OCH3)。
化合物2 白色胶状物,ESI-MS m/z: 251.2 [M + H]+。1H NMR (CD3OD, 500 MHz): δ 4.98 (1H, t, J = 10.0 Hz, H-6), 4.88 (1H, d, J = 9.6 Hz, H-1), 4.77 (1H, d, J = 10.0 Hz, H-5), 4.14 (1H, dd, J = 10.0, 6.0 Hz, H-3), 2.68 (1H, m, H-11), 2.34 (1H, m, H-9a), 2.33 (1H, m, H-2a), 2.28 (1H, m, H-2b), 2.21 (1H, m, H-7), 2.07 (1H, m, H-9a), 1.83 (1H, m, H-8a), 1.72 (3H, s, Me-15), 1.70 (1H, q, J = 13 Hz, H-8b), 1.45 (3H, s, Me-14), 1.23 (3H, q, J = 8.0 Hz, Me-13); 13C NMR(CD3OD, 125 MHz): δ 126.1 (d, C-1), 36.0 (t, C-2), 79.1 (d, C-3), 142.8 (s, C-4), 125.8 (d, C-5), 82.5 (d, C-6), 50.4 (d, C-7), 26.2 (t, C-8), 42.1 (t, C-9), 139.0 (s, C-10), 42.5 (d, C-11), 182.8 (s, C-12), 11.2 (q, C-13), 16.5 (q, C-14), 12.0 (q, C-15)。以上数据与文献[6]报道一致,故鉴定为3β-hydroxy-11α, 13-dihydro-costunolide。
化合物3 无色针状晶体(二氯甲烷),ESI-MS m/z: 305.1 [M + H]+。1H NMR (CDCl3, 500 MHz): δ 6.18 (1H, s, H-3), 4.83 (1H, td, J = 10.2, 1.5 Hz, H-8), 3.71 (1H, t, J = 10.0 Hz, H-6), 3.41 (1H, d, J = 10.0 Hz, H-5), 2.71 (1H, dd, J = 13.2, 11.0 Hz, Hα-9), 2.50 (1H, dd, J = 12.0, 7.0 Hz, H-11), 2.44 (3H, s, Me-14), 2.38 (1H, dd, J = 13.6, 2.0 Hz, Hβ-9), 2.32 (1H, ddd, J = 12.0, 10.0, 2.0 Hz, H-7), 2.30 (3H, s, Me-15), 2.11 (3H, s, Me-17), 1.34 (3H, d, J = 7.0 Hz, Me-13); 13C NMR (CDCl3, 125 MHz): δ 133.4 (s, C-1), 195.2 (s, C-2), 136.0 (d, C-3), 169.6 (s, C-4), 51.7 (d, C-5), 81.2 (d, C-6), 59.3 (d, C-7), 70.5 (d, C-8), 44.9 (t, C-9), 145.1 (s, C-10), 40.8 (d, C-11), 176.8 (s, C-12), 15.1 (q, C-13), 21.5 (q, C-14), 20.0 (q, C-15), 169.8 (s, C-16), 21.3 (q, C-17)。以上数据与文献[7]报道一致,故鉴定为matricarin。
化合物4 无色片状晶体(二氯甲烷),ESI-MS m/z: 469.4 [M + H]+。1H NMR (CDCl3, 400 MHz): δ 5.27 (1H, d, J = 6.8 Hz, H-21), 4.50 (1H, dd, J = 11.2, 6.8 Hz, H-21), 1.64, 1.06, 0.96, 0.89, 0.86, 0.85, 0.75 (each 3H, s, CH3×7), 0.85 (3H, d, J = 4.0 Hz, CH3-23), 2.04 (3H, s, CH3CO-); 13C NMR (CDCl3, 100 MHz): δ 38.4 (t, C-1), 23.7 (t, C-2), 81.0 (d, C-3), 37.8 (s, C-4), 55.4 (d, C-5), 18.2 (t, C-6), 34.2 (t, C-7), 41.1 (s, C-8), 50.3 (d, C-9), 37.0 (s, C-10), 21.6 (t, C-11), 27.0 (t, C-12), 39.2 (d, C-13), 42.3 (s, C-14), 27.6 (t, C-15), 36.7 (t, C-16), 34.4 (s, C-17), 48.7 (d, C-18), 36.3 (d, C-19), 139.8 (s, C-20), 118.9 (d, C-21), 42.2 (t, C-22), 27.9 (q, C-23), 16.4 (q, C-24), 16.0 (q, C-25), 16.5 (q, C-26), 14.7 (q, C-27), 21.6 (q, C-28), 17.7 (q, C-29), 22.5 (q, C-30), 171.0 (s, CH3CO-), 21.3 (q, CH3CO-)。以上数据与文献[8]报道一致,故鉴定为乙酰伪蒲公英甾醇。
化合物5 黄色油状物,ESI-MS m/z: 443.4 [M + H]+。1H NMR (CDCl3, 400 MHz): δ 4.78 (1H, d, J = 2.6 Hz, H-29a), 4.65 (1H, d, J = 2.6 Hz, H-29b), 3.20 (1H, dd, J = 11.0, 4.9 Hz, H-3), 1.70, 1.00, 0.96, 0.83, 0.79 (each 3H, s, CH3×5); 13C NMR (CDCl3, 100 MHz): δ 38.9 (t, C-1), 29.4 (t, C-2), 78.9 (d, C-3), 38.8 (s, C-4), 55.5 (d, C-5), 18.5 (t, C-6), 33.9 (t, C-7), 40.5 (s, C-8), 50.3 (d, C-9), 37.4 (s, C-10), 21.0 (t, C-11), 25.2 (t, C-12), 36.9 (d, C-13), 42.3 (s, C-14), 27.2 (t, C-15), 29.2 (t, C-16), 47.0 (s, C-17), 49.1 (d, C-18), 49.5 (d, C-19), 150.2 (s, C-20), 29.9 (t, C-21), 34.1 (t, C-22), 28.2 (q, C-23), 15.5 (q, C-24), 16.0 (q, C-25), 16.5 (q, C-26), 15.0 (q, C-27), 56.6 (t, C-28), 109.9 (t, C-29), 19.5 (q, C-30)。以上数据与文献[9]报道一致,故鉴定为白桦脂醇。
化合物6 无色针状晶体(二氯甲烷),ESIMS m/z: 473.4 [M + H]+。1H NMR (CDCl3, 400 MHz): δ 5.88 (1H, t, J = 2.5 Hz, H-12), 3.80, 3.20, (each 1H, d, J = 11.6 Hz, H-27a, 27b), 3.26 (1H, dd, J = 11.3, 4.9 Hz, H-3), 2.94 (1H, dd, J = 14.0, 4.5 Hz, H-18), 1.01, 1.00, 0.92, 0.90, 0.77, 0.75 (each 3H, s, CH3×6); 13C NMR (CDCl3, 100 MHz): δ 38.0 (t, C-1), 27.2 (t, C-2), 79.0 (d, C-3), 38.4 (s, C-4), 54.9 (d, C-5), 18.3 (t, C-6), 32.6 (t, C-7), 39.9 (s, C-8), 48.5 (d, C-9), 37.0 (s, C-10), 24.3 (t, C-11), 129.7 (d, C-12), 137.8 (s, C-13), 47.7 (s, C-14), 24.3 (t, C-15), 22.6 (t, C-16), 46.3 (s, C-17), 40.6 (d, C-18), 45.0 (t, C-19), 31.0 (s, C-20), 33.6 (t, C-21), 32.1 (t, C-22), 27.9 (q, C-23), 15.9 (q, C-24), 15.4 (q, C-25), 18.7 (q, C-26), 63.2 (t, C-27), 182.8 (s, C-28), 33.2 (q, C-29), 23.9 (q, C-30)。以上数据与文献[10]报道一致,故鉴定为27-hydroxylolean-12-en-28-oic acid。
化合物7 无色针状结晶(二氯甲烷),ESIMS m/z: 499.4 [M + H]+。1H NMR (CDCl3, 400 MHz): δ 5.23 (1H, s, H-19), 4.49 (1H, dd, J=10.8, 5.4 Hz, H-3), 2.05 (3H, s, -OCOCH3), 1.00, 0.99, 0.98, 0.89, 0.85, 0.84, 0.78 (each 3H, s, CH3×7); 13C NMR (CDCl3, 100 MHz): δ 38.5 (t, C-1), 23.8 (t, C-2), 80.8 (d, C-3), 37.7 (s, C-4), 55.5 (d, C-5), 18.1 (t, C-6), 33.3 (t, C-7), 40.3 (s, C-8), 51.2 (d, C-9), 37.3 (s, C-10), 21.0 (t, C-11), 26.1 (t, C-12), 41.3 (d, C-13), 42.6 (s, C-14), 29.5 (t, C-15), 33.7 (t, C-16), 48.0 (s, C-17), 137.1 (s, C-18), 133.6 (d, C-19), 32.1 (s, C-20), 33.4 (t, C-21), 34.5 (t, C-22), 28.0 (q, C-23), 16.9 (q, C-24), 15.9 (q, C-25), 16.6 (q, C-26), 15.0 (q, C-27), 171.3 (s, C-28), 30.5 (q, C-29), 29.1 (q, C-30), 171.3 (s, C-1′), 21.5 (q, C-2′)。以上数据与文献[11]报道一致,故鉴定为morolic acid acetate。
化合物8 无色针状结晶(二氯甲烷),ESIMS m/z: 197.1 [M + H]+。1H NMR (CDCl3, 600 MHz): δ 5.75 (1H, s, H-7), 4.22 (1H, m, H-3), 2.41 (1H, m, H-4b), 1.99 (1H, ddd, J = 14.4, 3.1, 2.3 Hz, H-2b), 1.76 (3H, s, H-11), 1.74 (1H, dd, J = 4.1, 0.9 Hz, H-4a), 1.53 (1H, dd, J = 14.5, 3.7 Hz, H-2a), 1.47 (3H, s, H-9), 1.28 (3H, s, H-10); 13C NMR (CDCl3, 150 MHz): δ 37.2 (s, C-1), 48.0 (t, C-2), 67.2 (d, C-3), 46.4 (t, C-4), 89.0 (s, C-5), 185.7 (s, C-6), 113.3 (d, C-7), 174.4 (s, C-8), 27.0 (q, C-9), 31.0 (q, C-10), 27.4 (q, C-11)。以上数据与文献[12–13]报道一致,故鉴定为异黑麦草内酯。
化合物9 无色油状物,ESI-MS m/z: 178.1 [M]+。1H NMR (CDCl3, 400 MHz): δ 8.08 (2H, m, H-2, 6), 7.72 (1H, dd, J = 5.6, 3.2 Hz, H-4), 7.53 (2H, m, H-3, 5), 4.30 (2H, t, J = 6.8 Hz, H-1′), 1.72 (2H, m, H-2′), 1.44 (2H, m, H-3′), 0.96 (3H, t, J = 7.2 Hz, H-4′); 13C NMR (CDCl3, 100 MHz): δ 132.4 (s, C-1), 129.9 (d, C-2, 6), 129.0 (d, C-3, 5), 131.1 (d, C-4), 65.7 (t, C-1′), 30.7 (t, C-2′), 19.3 (t, C-3′), 13.9 (t, C-4′), 167.9 (s, CO-)。以上数据与文献[14–15]报道一致, 故鉴定为苯甲酸丁酯。
化合物10 黄色油状物,ESI-MS m/z: 294.3 [M]+。1H NMR (CDCl3, 400 MHz): δ 9.99 (1H, d, J = 8.1 Hz, H-1), 5.88 (1H, ddd, J = 8.3, 2.7, 1.3 Hz, H-2), 2.16 (3H, s, H-17), 0.87 (6H, d, J = 2.3 Hz, H-16, 20), 0.85 (6H, d, J = 2.3 Hz, H-18, 19); 13C NMR (CDCl3, 100 MHz): δ 191.3 (d, C-1), 127.3 (d, C-2) 164.4 (s, C-3), 40.9 (t, C-4), 24.8 (t, C-5), 36.5 (t, C-6), 32.6 (d, C-7), 37.4 (t, C-8), 24.4 (t, C-9), 37.3 (t, C-10), 32.8 (d, C-11), 37.3 (t, C-12), 24.6 (t, C-13), 39.4 (t, C-14), 28.0 (d, C-15), 22.7 (q, C-16), 17.5 (q, C-17), 19.6 (q, C-18), 19.7 (q, C-19), 22.6 (q, C-20)。以上数据与文献[16–17]报道一致,故鉴定为E-phytenal。
1.4 抗菌活性测试采用牛津杯法对化合物1~10进行抑菌圈直径测试。LB培养基和马铃薯培养基(PDA)参照文献报道进行配制[18]。金黄色葡萄球菌和白色念珠菌分别置于LB培养基和PDA中活化,配制浓度为1× 107 CFU/mL的试验菌液。分别取上述菌液200 μL均匀涂布于相对应的培养基上,吸取浓度为20 mg/L (金黄色葡萄球菌抑菌圈直径测试)或2 mg/L (白色念珠菌抑菌圈直径测试)样品100 μL放入牛津杯中, 置于37 ℃环境下培养24 h,金黄色葡萄球菌和白色念珠菌分别以20 mg/L盐酸去甲万古霉素和2 mg/L重铬酸钾为阳性对照,以DMSO作为空白对照。抑菌圈直径 < 10 mm为不敏感,10~15 mm为中度敏感,15 mm以上为高度敏感。
采用二倍稀释法测定化合物的最小抗菌浓度(MIC)。96孔板中每孔加入10 μL被DMSO稀释成系列的药液(150.0、75.0、37.5、18.75、9.37、4.69、2.34、1.17 μg/mL),最后加入100 μL菌悬液接种。阳性对照分别为盐酸去甲万古霉素和重铬酸钾,以DMSO作为空白对照。96孔板置于37 ℃恒温箱内培养24 h,以肉眼观测完全没有菌落生长的最低浓度作为MIC值,重复实验3次。
从表 1, 2可见,化合物1、2、8具有一定抑制金黄色葡萄球菌生长的活性,抑菌圈直径和抑菌程度分别为10.2 (中敏感)、10.7 (中敏感)和11.0 mm (中敏感),MIC值分别为31.2、25.0、21.8 μg/mL。化合物1和3具有一定抑制白色念珠菌生长的活性,抑菌圈直径和抑菌程度分别为13.3 (中敏感)和10.0 mm (中敏感),MIC值为1.9和3.9 μg/mL。
| 表 1 化合物1~10对供试菌株抑菌圈直径(mm) Table 1 Ihibition zone diameters (mm) of compounds 1-10 |
| 表 2 化合物1~3、8最小抗菌浓度(μg/mL) Table 2 Minimal inhibitory concentration (MIC, μg/mL) of compounds 1-3 and 8 |
传统中药的药效物质基础已经得到了广泛的共识,以青蒿素为代表的一大批活性小分子或是以原型或是经过结构改造后应用于临床,如抗肿瘤、抗免疫、抗疟等治疗,因此,继续从传统中药中发掘新结构活性化合物具有重要的意义。本研究利用对民间药用兔耳风水提物的石油醚部位进行了系统化学成分研究,分离得到了10个单体化合物, 分别为松柏烯醇二异戊酸酯(1)、3β-hydroxy-11α, 13-dihydro-costunolide (2)、matricarin (3)、乙酰伪蒲公英甾醇(4)、白桦脂醇(5)、27-hydroxyolean-12-en-28-oic acid (6)、morolic acid acetate (7)、异黑麦草内酯(8)、苯甲酸丁酯(9)和E-phytenal (10)。其中化合物1和9为酚酸类成分,2和3为倍半萜类成分,4~7为三萜类成分,8为单萜类成分,10为长链脂肪醛类成分,这些成分类型和兔耳风属植物已报道的化学成分基本一致[19]。波谱分析结果表明,化合物1为新结构,化合物3和7首次在兔耳风属中分离得到,化合物2~7、9均首次在该植物中分离得到。化合物1、2、8对金黄色葡萄球菌(革兰氏阳性菌)具有一定的生长抑制活性,化合物1和3对白色念珠菌(真菌)有一定的抑制作用。这进一步丰富了该药材化合物种类,也为后续抗菌活性和药理活性的研究提供参考。
| [1] |
Delectis Florae Reipublicae Popularis Sinicae, Agendae Academiae Sinicae Editta. Florae Reipublicae Popularis Sinicae, Tomus 79[M]. Beijing: Science Press, 1996: 68-69. 中国科学院中国植物志编辑委员会. 中国植物志, 第79卷[M]. 北京: 科学出版社, 1996: 68-69. |
| [2] |
ZOU Y B, HE S Z. Species and distribution of Ainsliaea medicinal plants in Guizhou[J]. China J Chin Mat Med, 1998, 23(7): 389-392. 邹亚邦, 何顺志. 贵州省兔儿风属药用植物的种类与分布[J]. 中国中药杂志, 1998, 23(7): 389-392. |
| [3] |
CHEN X Y, HE W J, LIU Z, et al. Research progress on chemical constituents and pharmacological action of Ainsliaea fragrans Champ.[J]. Res Pract Chin Med, 2021, 35(6): 89-93. 陈欣悦, 贺文娟, 刘忠, 等. 杏香兔耳风化学成分及药理作用研究进展[J]. 现代中药研究与实践, 2021, 35(6): 89-93. DOI:10.13728/j.1673-6427.2021.06.018 |
| [4] |
CHEN Y P, WANG S L, SHEN Y H, et al. Chemical constituents from Ainsliaea glabra[J]. Guihaia, 2014, 34(3): 402-407. 陈亚萍, 王书林, 沈云亨, 等. 光叶兔儿风的化学成分研究[J]. 广西植物, 2014, 34(3): 402-407. DOI:10.3969/j.issn.1000-3142.2014.03.022 |
| [5] |
CHEN Y P, TONG C, LU W Q, et al. Three new sesquiterpenes from Ainsliaea glabra[J]. Nat Prod Res, 2019, 33(2): 274-279. DOI:10.1080/14786419.2018.1446134 |
| [6] |
GLASL S, MUCAJI P, WERNER I, et al. Sesquiterpenes and flavornoid aglycones from a Hungarian taxon of the Achillea millefolium group[J]. Z Naturforsch C J Biosci, 2002, 57(11/12): 976-982. DOI:10.1515/znc-2002-11-1203 |
| [7] |
AHMED A A, GÁTI T, HUSSEIN T A, et al. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1, 10-seco-guaianolide derivatives from Achillea ligustica[J]. Tetrahedron, 2003, 59(21): 3729-3735. DOI:10.1016/S0040-4020(03)00572-6 |
| [8] |
XU D, HOU F F, WU L J, et al. Chemical research of Dandelions[J]. China J Chin Mat Med, 2004, 29(3): 278. 许丹, 侯凤飞, 吴立军, 等. 蒲公英的化学研究[J]. 中国中药杂志, 2004, 29(3): 278. DOI:10.3321/j.issn:1001-5302.2004.03.029 |
| [9] |
DU K P, LI K J, GAO J, et al. The study on the antitumor effects and chemical components of the ethyl acetate extracts from Disporposis pernyi[J]. J Xuzhou Med Univ, 2019, 39(12): 868-871. 杜康彭, 李开金, 高剑, 等. 深裂竹根七乙酸乙酯提取物中的化学成分及抗肿瘤活性研究[J]. 徐州医科大学学报, 2019, 39(12): 868-871. DOI:10.3969/j.issn.2096-3882.2019.12.03 |
| [10] |
MAILLARD M, ADEWUNMI C O, HOSTETTMANN K, et al. A triterpene glycoside from the fruits of Tetrapleura tetraptera[J]. Phytochemistry, 1992, 31(4): 1321-1323. DOI:10.1016/0031-9422(92)80500-E |
| [11] |
LI N, YU F, YU S S, et al. Triterpenoids from Erythrophleum fordii[J]. J Integr Plant Biol, 2004, 46(3): 371-374. |
| [12] |
ZHOU W T, XIE H H. Phenylpropanoids, megastigmanes, alkaloid, and alkyl glycosides from Isodon serra[J]. J Trop Subtrop Bot, 2018, 26(2): 185-190. 周文婷, 谢海辉. 溪黄草的苯丙素、大柱香波龙烷、生物碱和烷基糖苷类成分[J]. 热带亚热带植物学报, 2018, 26(2): 185-190. DOI:10.11926/jtsb.3790 |
| [13] |
LIANG Y G, XU X Y, XIE H H, et al. Chemical constituents from Isodon lophanthoides var. graciliflora[J]. J Trop Subtrop Bot, 2010, 18(5): 564-568. 梁耀光, 徐新亚, 谢海辉, 等. 细花线纹香茶菜的化学成分研究[J]. 热带亚热带植物学报, 2010, 18(5): 564-568. DOI:10.3969/j.issn.1005-3395.2010.05.015 |
| [14] |
LYUTIKOVA M N, TUROV Y P. Chemical constituents from wild Oxycoccus palustris fruit from north Tyumen Oblast[J]. Chem Nat Compd, 2011, 46(6): 848-851. DOI:10.1007/s10600-011-9766-y |
| [15] |
HUANG S C, WU P L, WU T S, et al. Two coumarins from the root bark of Clausena excavata[J]. Phytochemistry, 1997, 44(1): 179-181. DOI:10.1016/S0031-9422(96)00532-8 |
| [16] |
RONTANI J F, CUNY P, GROSSI V. Photodegradation of chlorophyll phytyl chain in senescent leaves of higher plants[J]. Phytochemistry, 1996, 42(2): 347-351. DOI:10.1016/0031-9422(95)00872-1 |
| [17] |
WU J N, CHEN X T, SU J, et al. Chemical constituents of Bangia fuscopurpurea[J]. Chem Nat Compd, 2019, 55(3): 528-530. DOI:10.1007/s10600-019-02731-6 |
| [18] |
JIN L P. Investigation on bioactive secondary metabolites of dragonflies and termite' symbionts[D]. Jinhua: Zhejiang Normal University, 2017. 靳丽萍. 蜻蜓和白蚁共生菌的活性次生代谢产物研究[D]. 金华: 浙江师范大学, 2017. |
| [19] |
FENG F J, XU Z L, ZHANG Q J, et al. Advance on chemical compounds of Ainsliaea genus[J]. China J Chin Mat Med, 2015, 40(7): 1244-1251. 冯发进, 许志玲, 张前军, 等. 兔儿风属植物化学成分的研究进展[J]. 中国中药杂志, 2015, 40(7): 1244-1251. DOI:10.4268/cjcmm20150705 |
 2024, Vol. 32
2024, Vol. 32


