2. 中国科学院昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室, 昆明 650201;
3. 云南大学化学科学与工程学院, 昆明 650091
2. State Key Laboratory of Phytochemistry and Plant Resources in West China, and Yunnan Key Laboratory of Natural Medicinal Chemistry, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China;
3. School of Chemical Science and Technology, Yunnan University, Kunming 650091, China
植物内生菌是指在其生活史的一定阶段或全部阶段生活于健康植物的各种组织和器官的细胞间隙或细胞内的细菌或真菌。植物内生真菌是一类重要的微生物,通过产生具有广泛生物活性的次生代谢产物来增强宿主对非生物胁迫、疾病、昆虫、病原体、哺乳动物和食草动物的抵抗力[1]。滇重楼(Paris polyphylla var. Yunnanensis)为百合科(Liliaceae)重楼属植物,以根茎入药,具有清热解毒,消肿止痛,凉肝定经的功效[2]。作为传统中药,前人已对其化学成分进行了深入研究,而对滇重楼植物内生真菌的次生代谢产物研究则相对较少。烟曲霉(Aspergillus fumigatus)是植物中常见的一种内生真菌。目前,从内生真菌A. fumigatus中发现的次级代谢产物已经超过220多种[3],其主要的类型有酚类[4]、萜烯类[5]、异螺环-γ内酰胺类衍生物[6]、二酮哌嗪类衍生物[7]、其他生物碱类[8]和烟曲霉素类[9]等。然而,对来源于重楼属植物的内生真菌A. fumigatus研究较少,陈金印[10]在华重楼内生真菌的大米培养基发酵液中分离到麦角甾醇和过氧麦角甾醇,但是由于量过少未能对其化学成分开展深入的研究。
为进一步研究滇重楼内生真菌A. fumigatus中的次生代谢产物并比较其菌丝体和发酵液次生代谢产物的异同。本文采用液体发酵对滇重楼内生真菌A. fumigatus进行培养,并利用多种色谱分离技术和现代波谱技术,分别对其发酵液和菌丝体中的次生代谢产物进行了研究。
1 材料和方法 1.1 材料2020年11月3日刘海洋研究员于云南省文山壮族苗族自治州文山市采集标本,并鉴定为滇重楼(Paris polyphylla var. yunnanensis), 内生真菌通过PDA培养基从其块根中分离得到,通过形态和ITS基因测序鉴定为Aspergillus fumigatus (GenBank accession No. MZ054188.1),现保藏于云南大学化学科学与工程学院。
1.2 仪器和试剂HZP-250恒温培养摇床(上海精宏实验设备有限公司); Bruker AV Ⅲ 500核磁共振波谱仪和Bruker AV Ⅲ 600核磁共振波谱仪(德国Bruker公司);安捷伦1290 UPLC/6540 Q-TOF质谱仪(美国安捷伦公司);NU3000型半制备高效液相色谱仪(江苏汉邦仪器有限公司);Hei-VAP Value Digital型旋转蒸发仪(德国Heidolph公司);Sephadex LH-20 (瑞典Amersham Biosciences公司);Zorbax SB-C18半制备色谱柱(5 μm, 9.4 mm×250 mm; 美国安捷伦公司);300~ 400目柱层析硅胶(青岛海洋化工厂);薄层色谱硅胶板GF254 (临沂市海祥化工有限公司);乙酰胆碱酯酶、碘化硫代乙酰胆碱、DTNB、他克林(美国Sigma公司)、潮霉素B (美国Thermo Fisher Scientific公司)。
常规萃取提取分离用乙酸乙酯、甲醇、丙酮、三氯甲烷均为重蒸工业试剂; 氘代试剂均购自美国CIL (Cambridge Isotope Laboratories, Inc)公司;色谱乙腈购自上海星可高纯溶剂公司。
1.3 发酵、提取与分离用接种环挑取适量菌丝接种至装有200 mL PDB (200 g新鲜土豆,葡萄糖20 g,蒸馏水1 L)的500 mL三角瓶中,在恒温培养摇床(28 ℃, 150 r/min)中培养3 d, 得到菌株种子液。将等量种子液(每瓶20 mL)接种至含有200 mL PDB的1 L三角瓶中,在恒温培养摇床中培养7 d (28 ℃, 150 r/min),得到发酵培养液。
将菌株的发酵培养液离心,分为发酵液和菌丝体。取发酵液,用乙酸乙酯萃取4次,合并萃取液,得发酵液的乙酸乙酯萃取液。减压浓缩至干,得到该菌株发酵液的乙酸乙酯萃取物(粗提物4.0 g)。菌丝体用丙酮超声破碎提取3次,合并提取液,减压浓缩至不含丙酮后,用乙酸乙酯萃取4次,得菌丝体的乙酸乙酯萃取液。减压浓缩至干,得到菌丝体的乙酸乙酯萃取物(粗提物2.5 g)。
将发酵液粗提物经硅胶柱层析,以石油醚-乙酸乙酯(100:1~1:1)、氯仿-甲醇(10:1~1:1)梯度洗脱,得13个组分(Fr.1~Fr.13)。Fr.5经半制备HPLC (MeCN-H2O, 32:68, V/V, 下同)分离得到化合物15 (tR=14 min, 4.6 mg)。Fr.8经半制备HPLC (MeCN-H2O, 45:55)分离得到化合物5 (tR=19 min, 2.5 mg)、6 (tR=15 min, 3.2 mg)、7 (tR=22 min, 1.4 mg)和11 (tR=12 min, 3.6 mg)。Fr.9经Sephadex LH-20柱层析,由氯仿-甲醇(1:1)洗脱,得到组份Fr.9-1~Fr.9-4,Fr.9-1经半制备HPLC (MeOH-H2O, 36:64)分离得到化合物4 (tR=12 min, 3.6 mg)。Fr.9-2经半制备HPLC (MeCN-H2O, 30:70)分离得到化合物26 (tR=19 min, 43.8 mg)、27 (tR=11 min, 6.8 mg)和28 (tR=17 min, 9 mg)。Fr.9-3经半制备HPLC (MeCN-H2O, 35:65)分离得到化合物2 (tR=25 min, 5.7 mg)、8 (tR=14 min, 6.5 mg)、30 (tR=19 min, 17.0 mg)和31 (tR=10 min, 3.0 mg)。Fr.9-4经半制备HPLC (MeCN-H2O, 33:67)分离得到化合物1 (tR=15 min, 4.6 mg)。Fr.10经半制备HPLC (MeCN-H2O, 30:70)分离得到化合物3 (tR=17 min, 1.3 mg)、14 (tR=11 min, 6.9 mg)。Fr.11经半制备HPLC (MeCN-H2O, 31:69)分离得到化合物9 (tR=16 min, 14.8 mg)、13 (tR=13 min, 9.3 mg)和16 (tR=22 min, 5.4 mg)。Fr.12经半制备HPLC (MeCN-H2O, 34:66)分离得到化合物10 (tR= 12 min, 2.2 mg)和29 (tR=18 min, 47.5 mg)。Fr.13经半制备HPLC (MeCN-H2O, 34:66)分离得到化合物12 (tR=10 min, 2.5 mg)(图 1~3)。
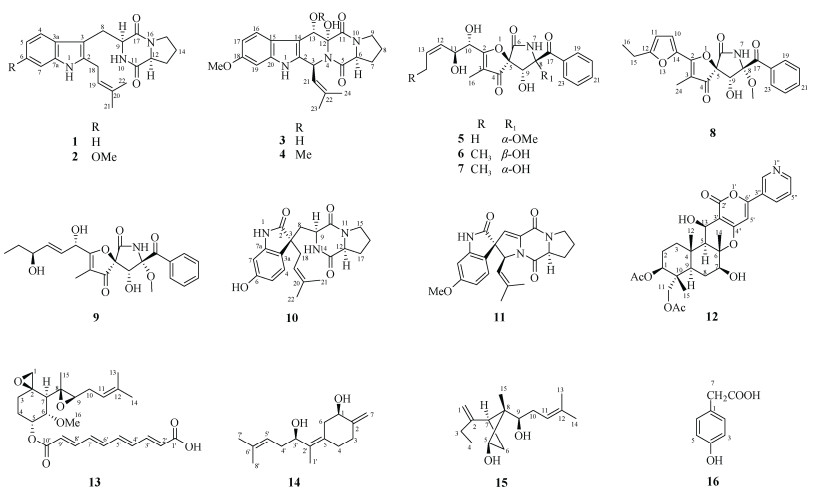
|
图 1 化合物1~16的结构 Fig. 1 Structures of compounds 1–16 |
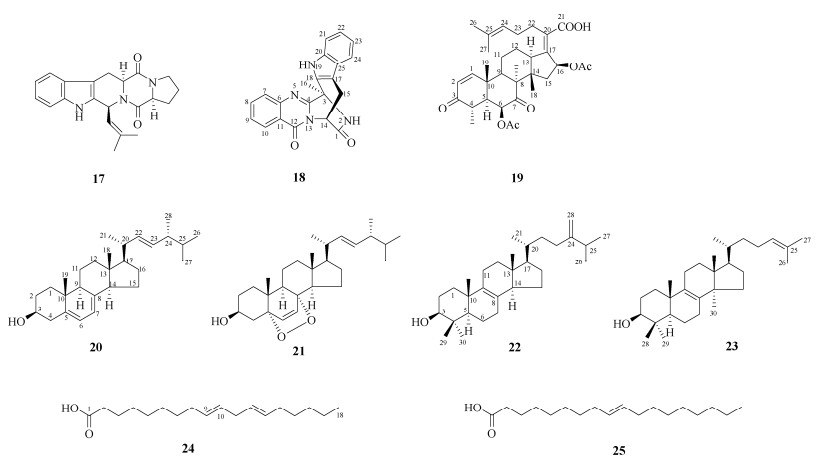
|
图 2 化合物17~25的结构 Fig. 2 Structures of compounds 17–25 |
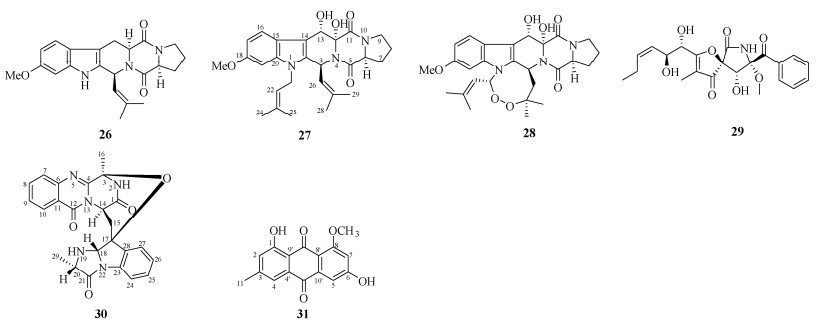
|
图 3 化合物26~31的结构 Fig. 3 Structures of compounds 26–31 |
将菌丝体粗提物经硅胶柱层析,以石油醚-乙酸乙酯(100:1~1:1)、氯仿-甲醇(10:1~1:1)梯度洗脱,得18个组分(Fr.1~Fr.18)。Fr.4经半制备HPLC (MeCN-H2O, 42:58)分离得到化合物22 (tR=14 min, 3.0 mg)和23 (tR=18 min, 1.0 mg)。Fr.5经半制备HPLC (MeCN-H2O, 55:45)分离得到化合物24 (tR=21 min, 7.0 mg)和25 (tR=16 min, 37.0 mg)。Fr.7通过结晶得到化合物20 (57.0 mg)。Fr.11经Sephadex LH-20柱层析,由氯仿-甲醇(1:1)洗脱,分离得到化合物21 (6.5 mg)。Fr.12经半制备HPLC (MeCN-H2O, 32:68)分离得到化合物17 (tR=14 min, 1.5 mg)、26 (tR=15 min, 55.7 mg)、27 (tR=10 min, 13.8 mg)和28 (tR=19 min, 16.6 mg)。Fr.13经Sephadex LH-20柱层析,由氯仿-甲醇(1:1)洗脱,分离得到化合物30 (17.0 mg)和31 (3.0 mg)。Fr.15经半制备HPLC (MeCN-H2O, 28:72)分离得到化合物19 (tR=13 min, 60.0 mg)。Fr.17经半制备HPLC (MeCN-H2O, 38:62)分离得到化合物18 (tR=10 min, 8.0 mg)。Fr.18经Sephadex LH-20柱层析, 由氯仿-甲醇(1:1)洗脱,分离得到化合物29 (14.7 mg)。
1.4 结构鉴定化合物1 淡黄色晶状固体,分子式C21H25N3O2。ESI-MS: m/z 352 [M + H]+。ESI-MS m /z: 281 [M + H]+。1H NMR (600 MHz, CDCl3): δH 7.97 (1H, s, NH-1), 7.49 (1H, d, J = 7.8 Hz, H-4), 7.31 (1H, d, J = 7.8 Hz, H-7), 7.16 (1H, t, J = 7.8 Hz, H-6), 7.09 (1H, t, J = 7.8 Hz, H-5), 5.62 (1H, s, H-10), 5.32 (1H, dd, J = 9.0, 7.2 Hz, H-19), 4.37 (1H, dd, J = 11.4, 4.2 Hz, H-9), 4.06 (1H, dd, J = 8.1, 6.9 Hz, H-12), 3.67 (2H, ddd, J = 12.8, 8.3, 3.6 Hz, H-15a, 8b), 3.59 (1H, ddd, J = 12.8, 8.9, 3.6 Hz, H-15b), 3.48 (1H, dd, J = 17.0, 9.0 Hz, H-18a), 3.44 (1H, dd, J = 17.0, 7.2 Hz, H-18b), 2.95 (1H, dd, J = 15.1, 11.4 Hz, H-8a), 2.32 (1H, m, H-13a), 2.03 (1H, m, H-13b), 1.98 (1H, m, H-14a), 1.89 (1H, m, H-14b), 1.79 (3H, s, CH3-21), 1.75 (3H, s, CH3-22); 13C NMR (150 MHz, CDCl3): δC 169.3 (C-11), 165.8 (C-17), 136.4 (C-2), 135.5 (C-20), 135.4 (C-7′), 128.0 (C-3′), 121.9 (C-6), 119.9 (C-5), 119.7 (C-19), 117.7 (C-4), 110.8 (C-7), 104.6 (C-2), 59.3 (C-12), 54.5 (C-9), 45.4 (C-15), 28.3 (C-13), 25.7 (C-21), 25.6 (C-8), 25.1 (C-18), 22.6 (C-14), 18.0 (C-22)。以上数据与文献[11]基本一致,故鉴定为tryprostatin B。
化合物2淡黄色晶状固体,分子式C22H27N3O3。ESI-MS: m/z 382 [M + H]+。1H NMR (500 MHz, CDCl3): δH 7.86 (1H, s, H-1), 7.33 (1H, d, J = 8.5 Hz, H-4), 6.82 (1H, d, J = 2.2 Hz, H-7), 6.76 (1H, dd, J = 8.5, 2.2 Hz, H-5), 5.63 (1H, s, H-10), 5.29 (1H, dd, J = 9.0, 7.5 Hz, H-19), 4.33 (1H, dd, J = 11.5, 4.0 Hz, H-9), 4.06 (1H, dd, J = 9.0, 7.0 Hz, H-12), 3.82 (3H, s, 6-OCH3), 3.67 (1H, ddd, J = 13.0, 8.5, 4.0 Hz, H-15a), 3.63 (1H, dd, J = 15.0, 4.0 Hz, H-8b), 3.58 (1H, ddd, J = 13.0, 9.0, 3.0 Hz, H-15b), 3.46 (1H, dd, J = 16.5, 9.0 Hz, H-18a), 3.40 (1H, dd, J = 16.5, 7.5 Hz, H-18b), 2.91 (1H, dd, J = 15.0, 11.5 Hz, H-8a), 2.33 (1H, m, H-13a), 2.05 (2H, m, H-13b, H-14a), 1.94 (1H, m, H-14b), 1.77 (3H, s, CH3-21), 1.74 (3H, s, CH3-22); 13C NMR (125 MHz, CDCl3): δC 169.3 (C-11), 165.8 (C-17), 156.4 (C-6), 136.3 (C-7′), 135.3 (C-20), 135.1 (C-2), 122.3 (C-4), 120.0 (C-19), 118.4 (C-5), 109.4 (C-5), 104.5 (C-3), 94.9 (C-7), 59.3 (C-12), 55.8 (6-OCH3), 54.6 (C-9), 45.4 (C-15), 28.4 (C-13), 25.8 (C-21), 25.7 (C-8), 25.1 (C-18), 22.7 (C-14), 18.0 (C-22)。以上数据与文献[11]基本一致,故鉴定为tryprostatin A。
化合物3 黄色晶状固体,分子式C22H25N3O5。ESI-MS m /z: 412 [M + H]+。1H NMR (600 MHz, CDCl3): δH 7.80 (1H, d, J = 8.7 Hz, H-16), 7.66 (1H, s, NH-1), 6.85 (1H, d, J = 2.2 Hz, H-19), 6.81 (1H, dd, J = 8.7, 2.2 Hz, H-17), 5.88 (1H, d, J = 9.6 Hz, H-3), 4.81 (1H, m, H-21), 4.43 (1H, dd, J = 9.9, 6.6 Hz, H-6), 3.83 (3H, s, 18-OCH3), 3.66 (1H, m, H-9a), 3.64 (1H, m, H-9b), 2.49 (1H, m, H-7a), 2.09 (2H, m, H-7b, 8a), 2.01 (3H, s, CH3-24), 1.67 (3H, s, CH3-23); 13C NMR (150 MHz, CDCl3): δC 171.0 (C-11), 166.1 (C-5), 156.7 (C-18), 137.5 (C-20), 134.6 (C-22), 130.1 (C-2), 124.0 (C-21), 121.2 (C-16), 120.7 (C-15), 109.9 (C-17), 105.3 (C-14), 95.0 (C-19), 83.0 (C-12), 68.6 (C-13), 58.7 (C-3), 55.7 (18-OCH3), 50.1 (C-6), 45.3 (C-9), 29.1 (C-7), 25.7 (C-23), 22.5 (C-8), 18.3 (C-24)。以上数据与文献[12]基本一致,故鉴定为12, 13-dihydroxyfumitremorgin C。
化合物4 淡黄色晶状固体,分子式C23H27N3O5。ESI-MS m/z: 426 [M + H]+。1H NMR (600 MHz, CDCl3): δH 7.82 (1H, s, NH-1), 7.44 (1H, d, J = 8.7 Hz, H-16), 6.88 (1H, d, J = 2.2 Hz, H-19), 6.82 (1H, dd, J = 8.7, 2.2 Hz, H-17), 6.64 (1H, d, J = 9.9 Hz, H-3), 5.56 (1H, d, J = 9.9 Hz, H-21), 4.73 (1H, s, H-13), 4.39 (1H, dd, J = 10.5, 6.3 Hz, H-6), 3.83 (3H, s, 18-OCH3), 3.75 (1H, m, H-9a), 3.70 (1H, m, H-9b), 3.36 (3H, s, 13-OCH3), 2.50 (1H, m, H-7a), 2.11 (1H, m, H-8b), 2.06 (3H, d, J = 1.4 Hz, CH3-24), 2.00 (2H, m, H-7b, 8a), 1.79 (3H, d, J = 1.4 Hz, CH3-23); 13C NMR (150 MHz, CDCl3): δC 167.0 (C-5), 165.8 (C-11), 156.5 (C-18), 137.9 (C-22), 136.6 (C-20), 133.7 (C-2), 123.5 (C-21), 122.6 (C-15), 118.6 (C-16), 110.0 (C-17), 105.3 (C-14), 95.2 (C-19), 84.7 (C-12), 76.8 (C-13), 60.0 (C-6), 56.6 (13-OCH3), 55.7 (18-OCH3), 49.1 (C-3), 45.8 (C-9), 29.7 (C-7), 26.1 (C-23), 22.1 (C-8), 18.2 (C-24)。以上数据与文献[13]基本一致,故鉴定为cyclotryprostatin B。
化合物5 淡黄色粉末,分子式C21H23NO8。ESI-MS m/z: 440 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 8.32 (2H, d, J = 7.8 Hz, H-19, 23), 7.65 (1H, t, J = 7.8 Hz, H-21), 7.49 (2H, t, J = 7.8 Hz, H-20, 22), 5.72 (1H, dq, J = 13.8, 7.2 Hz, H-13), 5.37 (1H, t, J = 10.2 Hz, H-12), 4.78 (1H, s, H-11), 4.69 (1H, d, J = 11.1 Hz, H-9), 4.61 (1H, d, J = 5.4 Hz, H-10), 3.42 (3H, s, 8-OCH3), 1.73 (3H, d, J = 7.2 Hz, CH3-15), 1.69 (3H, s, CH3-16); 13C NMR (150 MHz, CDCl3): δC 196.4 (C-4), 194.9 (C-17), 185.7 (C-2), 166.5 (C-6), 134.8 (C-21), 132.3 (C-18), 130.7 (C-23), 130.7 (C-19), 129.6 (C-13), 128.7 (C-22), 128.7 (C-20), 128.0 (C-12), 113.6 (C-3), 92.7 (C-5), 90.2 (C-8), 73.2 (C-9), 70.4 (C-11), 70.3 (C-10), 51.8 (8-OCH3), 13.7 (C-15), 6.0 (C-16)。以上数据与文献[14]基本一致,故鉴定为14-norpseurotin A。
化合物6 白色粉末,分子式C21H23NO8。ESI-MS m/z: 440 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 8.64 (1H, s, NH-1), 8.38 (2H, d, J = 7.8 Hz, H-19, 23), 7.48 (3H, m, H-20, 21, 22), 5.54 (1H, d, J = 10.8 Hz, H-13), 5.14 (1H, t, J = 10.8 Hz, H-12), 4.80 (1H, m, H-9), 4.74 (1H, d, J = 7.2 Hz, H-11), 2.09 (1H, dd, J = 14.1, 7.2 Hz, H-14), 1.62 (3H, s, CH3-16), 0.98 (3H, t, J = 7.2 Hz, CH3-15); 13C NMR (150 MHz, CDCl3): δC 198.8 (C-4), 194.0 (C-17), 188.9 (C-6), 165.4 (C-2), 136.6 (C-13), 134.6 (C-21), 132.9 (C-18), 131.6 (C-19), 128.5 (C-20), 125.1 (C-12), 113.0 (C-3), 94.7 (C-5), 89.4 (C-8), 71.7 (C-11), 71.6 (C-9), 70.8 (C-10), 21.4 (C-14), 14.0 (C-15), 6.3 (C-16)。以上数据与文献[15]基本一致,故鉴定为pseurotine F1。
化合物7 白色粉末,分子式C21H23NO8。ESI-MS m/z: 440 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 9.48 (1H, s, NH-1), 8.30 (2H, d, J = 7.8 Hz, H-19, 23), 7.48 (3H, m, H-20, 21, 22), 5.50 (1H, d, J = 10.8 Hz, H-13), 5.06 (1H, t, J = 9.9 Hz, H-12), 4.83 (1H, d, J = 7.2 Hz, H-11), 4.72 (1H, m, H-9), 2.09 (1H, dd, J = 14.1, 7.2 Hz, H-14), 1.68 (3H, s, CH3-16), 0.99 (3H, t, J = 7.2 Hz, CH3-15); 13C NMR (150 MHz, CDCl3): δC 199.9 (C-4), 194.4 (C-17), 188.9 (C-6), 167.6 (C-2), 136.0 (C-13), 134.6 (C-21), 133.2 (C-18), 130.7 (C-19), 128.7 (C-20), 125.3 (C-12), 112.0 (C-3), 93.8 (C-8), 92.7 (C-5), 77.4 (C-9), 72.6 (C-11), 71.2 (C-10), 21.4 (C-14), 14.0 (C-15), 6.6 (C-16)。以上数据与文献[15]基本一致,故鉴定为pseurotine F2。
化合物8 淡黄色油状,分子式C22H21NO7。ESI-MS m/z: 434 [M + Na]+。1H NMR (500 MHz, CDCl3): δH 8.32 (2H, d, J = 8.0 Hz, H-19, 23), 7.63 (1H, t, J = 8.0 Hz, H-21), 7.48 (2H, m, H-20, 22), 7.03 (1H, d, J = 3.5 Hz, H-10), 6.22 (1H, d, J = 3.5 Hz, H-11), 4.69 (1H, d, J = 12.5 Hz, H-9), 3.40 (3H, s, CH3-25), 2.74 (2H, q, J = 7.5 Hz, H-15), 2.01 (3H, s, CH3-24), 1.27 (3H, t, J = 7.5 Hz, CH3-16); 13C NMR (125 MHz, CDCl3): δC 195.7 (C-4), 194.7 (C-17), 172.6 (C-2), 166.2 (C-6), 163.8 (C-12), 143.4 (C-14), 134.6 (C-21), 132.5 (C-18), 130.6 (C-19, 23), 128.7 (C-20, 22), 118.3 (C-10), 107.9 (C-3), 107.8 (C-11), 91.6 (C-5), 89.7 (C-8), 74.2 (C-9), 51.7 (C-25), 21.8 (C-15), 11.8 (C-16), 6.2 (C-24)。以上数据与文献[16]基本一致,故鉴定为azaspirofuran A。
化合物9 黄色粉末,分子式C22H25NO8。ESI-MS m/z: 454 [M + Na]+。1H NMR (600 MHz, CD3OD): δH 8.34 (2H, d, J = 7.8 Hz, H-19, 23), 7.63 (1H, t, J = 7.8 Hz, H-21), 7.49 (2H, t, J = 7.8 Hz, H-20, 22), 5.86 (1H, m, H-12), 5.80 (1H, m, H-11), 5.17 (1H, t, J = 5.4 Hz, H-10), 4.49 (1H, d, J = 9.0 Hz, H-6), 3.99 (1H, m, H-13), 3.33 (3H, s, 8-OCH3), 1.75 (3H, s, CH3-16), 0.90 (3H, t, J = 7.2 Hz, CH3-15); 13C NMR (150 MHz, CD3OD): δC 199.2 (C-4), 197.1 (C-17), 189.1 (C-6), 168.8 (C-2), 137.9 (C-12), 135.1 (C-21), 134.9 (C-18), 131.8 (C-19, 23), 129.5 (C-20, 22), 128.0 (C-11), 112.1 (C-3), 93.8 (C-8), 93.6 (C-5), 76.3 (C-9), 74.0 (C-10), 69.8 (C-13), 52.4 (8-OCH3), 30.9 (C-14), 10.1 (C-15), 5.5 (C-16)。以上数据与文献[17]基本一致,故鉴定为pseurotin D。
化合物10 白色粉末。分子式C21H25N3O4。ESI-MS m/z: 406 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 7.84 (1H, s, 14-NH), 7.10 (1H, d, J = 8.1 Hz, H-4), 6.57 (1H, dd, J = 8.1, 2.4 Hz, H-5), 6.54 (1H, d, J = 2.4 Hz, H-7), 4.10 (1H, dd, J = 8.1, 2.1 Hz, H-9), 3.87 (1H, t, J = 8.1 Hz, H-12), 3.65 (1H, dd J =15.0, 2.1 Hz, H-8a), 3.50 (1H, m, H-15a), 3.36 (1H, m, H-15b), 2.51 (1H, dd, J =15.0, 8.1 Hz, H-8b), 1.62 (1H, m, H-16a), 1.60 (3H, s, CH3-22), 1.53 (1H, m, H-16b), 1.49 (3H, s, CH3-21); 13C NMR (150 MHz, CDCl3): δC 182.6 (C-2), 169.9 (C-10), 165.2 (C-13), 160.2 (C-6), 141.7 (C-7′), 136.4 (C-20), 124.7 (C-4), 122.3 (C-3′), 116.7 (C-19), 107.5 (C-5), 97.3 (C-7), 58.9 (C-12), 55.5 (C-9), 52.6 (C-3), 45.6 (C-15), 37.7 (C-18), 35.0 (C-8), 27.9 (C-17), 25.9 (C-21), 22.8 (C-16), 18.0 (C-22)。以上数据与文献[18]基本一致,故鉴定为spirotryprostatin K。
化合物11 黄色粉末,分子式C22H23N3O4。ESI-MS m/z: 416 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 6.95 (1H, d, J = 8.4 Hz, H-4), 6.51 (1H, dd, J = 8.4, 2.4 Hz, H-5), 6.44 (1H, d, J = 2.4 Hz, H-7), 5.75 (1H, s, H-8), 5.37 (1H, d, J = 9.0 Hz, H-18), 5.18 (1H, d, J = 9.0 Hz, H-19), 4.33 (1H, dd, J = 10.5, 6.3 Hz, H-12), 3.83 (1H, m, H-15b), 3.80 (3H, s, 6-OCH3), 3.56 (1H, m, H-15a), 2.47 (1H, m, H-17a), 2.11 (1H, m, H-16b), 1.99 (1H, m, H-17b), 1.97 (1H, m, H-16a), 1.59 (3H, s, CH3-21), 1.29 (3H, s, CH3-22); 13C NMR (150 MHz, CDCl3): δC 178.6 (C-2), 162.5 (C-13), 160.6 (C-6), 155.2 (C-10) 141.5 (C-7′), 138.3 (C-20), 137.9 (C-9), 128.5 (C-4), 120.4 (C-19), 118.9 (C-3′), 116.8 (C-8), 107.1 (C-5), 97.1 (C-7), 64.0 (C-18), 61.5 (C-12), 61.3 (C-3), 55.5 (6-OCH3), 44.8 (C-15), 29.2 (C-17), 25.4 (C-21), 22.1 (C-16), 18.3 (C-22)。以上数据与文献[14]基本一致,故鉴定为6-methoxyspirotryprostatin B。
化合物12 白色粉末,分子式C29H35NO9。ESI-MS m/z: 542 [M + H]+。1H NMR (600 MHz, CDCl3): δH 9.03 (1H, d, J = 2.4 Hz, H-2′′), 8.72 (1H, dd, J = 4.8, 1.8 Hz, H-6′′), 8.13 (1H, dt, J = 8.1, 1.8 Hz, H-4′′), 7.44 (1H, dd, J = 8.1, 4.8 Hz, H-5′), 5.01 (1H, d, J = 4.2 Hz, H-13), 4.99 (1H, dd, J = 12.3, 5.4 Hz, H-7), 4.81 (1H, dd, J = 11.7, 4.8 Hz, H-1), 3.84 (1H, d, J = 10.2 Hz, H-11a), 3.82 (1H, d, J = 10.2 Hz, H-11b), 2.18 (1H, td, J = 12.6, 4.8 Hz, H-3a), 2.00 (3H, s, 1-OAc), 1.99 (3H, s, 11-OAc), 1.91 (1H, m, H-2a), 1.85 (1H, m, H-2b), 1.81 (1H, m, H-8b), 1.68 (3H, s, CH3-14), 1.66 (1H, d, J = 3.6 Hz, H-5), 1.51 (1H, d, J = 12.3 Hz, H-8a), 1.49 (1H, d, J = 4.2 Hz, H-9), 1.44 (3H, s, CH3-12), 1.39 (1H, dt, J = 12.6, 7.2 Hz, H-3b), 0.93 (3H, s, CH3-15); 13C NMR (150 MHz, CDCl3): δC 170.8 (OAc-11), 170.6 (OAc-1), 164.0 (C-2′), 162.3 (C-2′), 157.4 (C-4′), 150.6 (C-6′′), 146.8 (C-2′′), 133.0 (C-4′′), 127.2 (C-6′), 127.0 (C-3′′), 123.7 (C-5′′), 103.1 (C-5′), 99.2 (C-3′), 85.5 (C-6), 77.6 (C-7), 73.5 (C-1), 65.1 (C-11), 60.3 (C-13), 54.3(C-5), 45.5 (C-9), 40.4 (C-10), 38.1 (C-4), 36.1 (C-3), 27.4 (C-8), 22.7 (C-2), 21.2 (OAc-1), 20.9 (OAc-11), 17.6 (C-12), 15.4 (C-14), 13.1 (C-15)。以上数据与文献[19]基本一致,故鉴定为7-deacetylpyripyropene A。
化合物13 无色粉末,分子式C26H34O7。ESI-MS m/z: 457 [M – H]–。1H NMR (500 MHz, CDCl3): δH 7.36 (1H, dd, J = 12.0, 15.0 Hz, H-8′), 7.31 (1H, dd, J = 12.0, 15.0 Hz, H-3′), 6.82 (1H, m, H-6′), 6.80 (1H, m, H-5′), 6.61 (2H, m, H-4′, H-7′), 6.00 (1H, d, J = 15.0 Hz, H-9′), 5.94 (1H, d, J = 15.0 Hz, H-2′), 5.70 (1H, q, J = 3.0 Hz, H-5), 5.20 (1H, m, H-11), 3.68 (1H, m, H-6), 3.43 (3H, s, 16-OCH3), 2.99 (1H, d, J = 4.5 Hz, H-1b), 2.63 (1H, m, H-9), 2.55 (1H, d, J = 4.5 Hz, H-1a), 2.21 (2H, m, H-10), 2.10 (1H, m, H-3a), 1.97 (1H, m, H-7), 1.90 (2H, m, H-4), 1.73 (3H, s, H-13), 1.65 (3H, s, CH3-14), 1.21 (3H, s, CH3-15), 1.10 (1H, m, H-3b); 13C NMR (125 MHz, CDCl3): δC 171.8 (C-1′), 167.6 (C-10′), 146.9 (C-8′), 144.9 (C-3′), 141.4 (C-6′), 140.2 (C-5′), 136.4 (C-12), 135.6 (C-7′), 134.7 (C-4′), 124.8 (C-2′), 123.3 (C-9′), 119.9 (C-11), 80.7 (C-6), 67.9 (C-5), 62.4 (C-9), 60.9 (C-8), 60.2 (C-2), 58.1 (16-OCH3), 52.3 (C-1), 49.6 (C-7), 30.8 (C-3), 28.8 (C-4), 27.1 (C-10), 27.1 (C-13), 19.4 (C-14), 15.3 (C-15)。以上数据与文献[20]基本一致,故鉴定为烟曲霉素。
化合物14 黄色固体,分子式C15H24O2。ESI-MS m/z: 259 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 5.08 (1H, m, H-5′), 4.92 (1H, s, H-7b), 4.81 (1H, s, H-7a), 4.63 (1H, t, J = 7.2 Hz, H-3′), 4.15 (1H, dd, J = 6.9, 3.6 Hz, H-1), 2.53 (1H, m, H-6b), 2.45 (1H, m, H-6a), 2.42 (1H, m, H-3b), 2.35 (1H, m, H-4′b), 2.15 (3H, m, H-3a, 4a, 4′a), 1.73 (3H, s, CH3-1′), 1.71 (3H, s, CH3-7′), 1.64 (3H, s, CH3-8′); 13C NMR (150 MHz, CDCl3): δC 150.1 (C-2), 134.4 (C-6′), 131.1 (C-2′), 131.0 (C-5), 120.2 (C-5′), 108.0 (C-7), 73.1 (C-1), 69.8 (C-3′), 38.5 (C-6), 33.6 (C-4′), 32.3 (C-3), 31.5 (C-4), 25.9 (C-7′), 18.0 (C-8′), 11.8 (C-1′)。以上数据与文献[21]基本一致,故鉴定为fumagillene A。
化合物15 无色粉末,分子式C15H24O2。ESI-MS: m/z 219 [M + H ‒ H2O]+。1H NMR (600 MHz, CDCl3): δH 5.25 (1H, t, J = 6.9 Hz, H-11), 4.69 (1H, s, H-1a), 4.58 (1H, s, H-1b), 4.32 (1H, dd, J = 9.9, 2.4 Hz, H-9), 2.62 (1H, m, H-3a), 2.43 (1H, ddd, J = 9.6, 6.6, 2.1 Hz, H-6b), 2.37 (1H, d, J = 6.6 Hz, H-7), 2.33 (1H, d, J = 9.6 Hz, H-3b), 2.06 (2H, dd, J = 14.5, 4.8 Hz, H-10), 1.95 (1H, m, H-4b), 1.90 (1H, d, J = 9.9 Hz, H-6a), 1.82 (1H, ddd, J = 12.9, 11.4, 1.5 Hz, H-4a), 1.76 (3H, s, CH3-13), 1.67 (3H, s, CH3-14), 0.85 (3H, s, CH3-15); 13C NMR (150 MHz, CDCl3): δC 148.0 (C-2), 135.3 (C-12), 121.0 (C-11), 107.7 (C-1), 76.6 (C-5), 74.3 (C-9), 52.4 (C-8), 42.1 (C-7), 36.4 (C-6), 31.4 (C-4), 31.2 (C-10), 25.9 (C-13), 25.2 (C-3), 18.0 (C-14), 11.5 (C-15)。以上数据与文献[22]基本一致,故鉴定为5, 9-dihydroxy-β-trans-bergamotene。
化合物16 无色粉末,分子式C8H8O3。ESI-MS m/z: 151 [M – H]–。1H NMR (600 MHz, CD3OD): δH 7.10 (2H, d, J = 8.4 Hz, H-3, H-5), 6.72 (2H, d, J = 8.4 Hz, H-2, H-6), 3.39 (2H, s, H-7); 13C NMR (150 MHz, CD3OD): δC 177.7 (C-8), 157.5 (C-4), 131.3 (C-3), 131.2 (C-5), 127.6 (C-1), 116.3 (C-2), 116.2 (C-6), 42.6 (C-7)。以上数据与文献[23]基本一致, 故鉴定为对羟基苯乙酸。
化合物17 黄色粉末,分子式C21H23N3O2。ESI-MS m/z: 372 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 7.79 (1H, s, H-1), 7.58 (1H, d, J = 7.8 Hz, H-16), 7.35 (1H, d, J = 7.8 Hz, H-19), 7.19 (1H, t, J = 7.8 Hz, H-18), 7.15 (1H, t, J = 7.8 Hz, H-17), 6.03 (1H, d, J = 9.6 Hz, H-3), 4.92 (1H, d, J = 9.6 Hz, H-21), 4.20 (1H, dd, J = 11.7, 4.9 Hz, H-12), 4.13 (1H, t, J = 8.1 Hz, H-6), 3.66 (1H, m, H-9a), 3.58 (1H, dd, J = 15.9, 4.9 Hz, H-13a), 3.13 (1H, dd, J = 15.9, 11.7 Hz, H-13b), 2.42 (1H, m, H-7a), 2.35 (1H, t, J = 7.5 Hz, H-9b), 2.25 (1H, m, H-7b), 2.06 (1H, m, H-8a), 2.02 (3H, s, CH3-24), 1.94 (1H, m, H-8b), 1.62 (3H, s, CH3-23); 13C NMR (150 MHz, CDCl3): δC 169.5 (C-5), 165.7 (C-11), 136.1 (C-20), 134.3 (C-22), 133.5 (C-2), 125.3 (C-15), 124.0 (C-21), 122.2 (C-18), 120.1 (C-17), 118.3 (C-16), 111.1 (C-19), 106.5 (C-14), 59.2 (C-6), 56.8 (C-12), 51.0 (C-3), 45.4 (C-9), 28.6 (C-7), 25.7 (C-23), 23.1 (C-8), 21.9 (C-13), 18.2 (C-24)。以上数据与文献[11]基本一致,故鉴定为demethoxyfumitremorgin C。
化合物18 白色粉末,分子式C21H16N4O2。ESI-MS m/z: 357 [M + H]+。1H NMR (600 MHz, Acetone-d6): δH 8.47 (1H, s, H-19), 8.20 (1H, dd, J = 8.1, 1.5 Hz, H-10), 7.78 (1H, ddd, J = 8.1, 7.2, 1.5 Hz, H-8), 7.64 (1H, dd, J = 8.1, 1.5 Hz, H-7), 7.51 (1H, ddd, J = 8.1, 7.2, 1.5 Hz, H-9), 7.46 (1H, dt, J = 8.1, 1.2 Hz, H-24), 7.35 (1H, d, J = 8.1 Hz, H-21), 7.11 (1H, ddd, J = 8.1, 7.2, 1.2 Hz, H-22), 7.01 (1H, ddd, J = 8.1, 7.2, 1.2 Hz, H-23), 5.88 (1H, ddd, J = 4.5, 2.7, 1.5 Hz, H-14), 3.50 (1H, dd, J = 17.1, 2.7 Hz, H-15b), 3.34 (1H, dd, J = 17.1, 4.5 Hz, H-15a), 2.29 (3H, s, CH3-16); 13C NMR (150 MHz, Acetone-d6): δC 170.1 (C-1), 160.5 (C-12), 155.5 (C-4), 148.2 (C-6), 136.1 (C-20), 135.3 (C-8), 134.9 (C-18), 128.9 (C-25), 128.4 (C-9), 127.8 (C-7), 127.3 (C-10), 123.4 (C-22), 121.7 (C-23), 120.6 (C-11), 119.0 (C-24), 112.4 (C-21), 107.3 (C-17), 55.8 (C-3), 55.5 (C-14), 26.7 (C-15), 18.7 (C-16)。以上数据与文献[24]基本一致,故鉴定为fumiquinazoline J。
化合物19 白色粉末,分子式C33H44O8。ESI-MS m/z: 591 [M + Na]+。1H NMR (500 MHz, CDCl3): δH 7.30 (1H, d, J = 10.0 Hz, H-1), 5.97 (1H, d, J = 8.0 Hz, H-16), 5.86 (1H, d, J = 10.0 Hz, H-2), 5.22 (1H, s, H-6), 5.10 (1H, t, J = 7.0 Hz, H-24), 2.77 (1H, m, H-4), 2.61 (1H, dd, J = 13.0, 3.0 Hz, H-9), 2.55 (1H, d, J = 12.0 Hz, H-13), 2.49 (2H, m, H-22), 2.47 (1H, m, H-4), 2.44 (1H, m, H-12a), 2.26 (1H, d, J = 12.5 Hz, H-5), 2.19 (1H, m, H-15a), 2.11 (3H, s, 6-OAc), 2.08 (1H, m, H-23b), 2.06 (1H, m, H-23a), 1.99 (1H, m, H-11a), 1.96 (3H, s, 16-OAc), 1.87 (1H, d, J = 14.5 Hz, H-15b), 1.76 (1H, m, H-12b), 1.68 (3H, s, CH3-26), 1.60 (3H, s, CH3-27), 1.55 (1H, m, H-11b), 1.44 (3H, s, CH3-19), 1.27 (3H, d, J = 6.8 Hz, CH3-28), 1.16 (3H, s, CH3-8), 0.93 (3H, s, CH3-18); 13C NMR (125 MHz, CDCl3): δC 209.0 (C-7), 201.4 (C-3), 173.4 (C-21), 170.2 (OAc-16), 168.9 (OAc-6), 157.3 (C-1), 147.8 (C-17), 132.2 (C-25), 130.0 (C-20), 127.8 (C-2), 123.2 (C-24), 73.8 (C-6), 73.4 (C-16), 52.7 (C-8), 49.2 (C-13), 47.2 (C-5), 46.7 (C-14), 41.7 (C-9), 40.7 (C-4), 40.4 (C-15), 38.2 (C-10), 28.9 (C-22), 28.2 (C-23), 27.5 (C-19), 25.9 (C-12), 25.8 (C-27), 24.0 (C-11), 20.8 (OAc-6), 20.8 (OAc-16), 18.3 (C-29), 17.9 (C-18), 17.8 (C-26), 13.1 (C-28)。以上数据与文献[25]基本一致,故鉴定为烟曲霉酸。
化合物20 无色晶体,分子式C28H44O。ESI-MS m/z: 419 [M + Na]+。1H NMR (500 MHz, CDCl3): δH 5.57 (1H, dd, J = 5.5, 2.5 Hz, H-6), 5.38 (1H, dt, J = 5.5, 2.5 Hz, H-7), 5.20 (1H, dd, J = 15.5, 7.5 Hz, H-23), 5.13 (1H, dd, J = 15.5, 7.5 Hz, H-22), 3.64 (1H, tt, J = 11.0, 4.5 Hz, H-3), 2.47 (1H, ddd, J = 14.5, 4.5, 2.5 Hz, H-4a), 2.29 (1H, ddt, J = 14.5, 11.0, 2.5 Hz, H-4b), 2.10 (1H, m, H-12), 1.98 (1H, m, H-9), 1.95 (1H, m, H-14), 1.90 (1H, m, H-1), 1.84 (1H, m, H-2a), 1.83 (1H, m, H-16), 1.78 (1H, m, H-11), 1.67 (1H, m, H-15), 1.65 (1H, m, H-11), 1.50 (1H, m, H-25), 1.48 (1H, m, H-2b), 1.46 (1H, m, H-15), 1.45 (1H, m, H-16), 1.37 (1H, m, H-17), 1.34 (1H, m, H-12), 1.26 (1H, m, H-1), 1.04 (3H, d, J = 6.5 Hz, CH3-21), 0.95 (3H, s, CH3-19), 0.92 (3H, d, J = 6.5 Hz, CH3-28), 0.85 (3H, d, J = 6.5 Hz, CH3-27), 0.83 (3H, dd, J = 7.5, 7.0 Hz, CH3-26), 0.63 (3H, s, CH3-18); 13C NMR (125 MHz, CDCl3): δC 141.4 (C-8), 139.8 (C-5), 135.6 (C-23), 132.0 (C-22), 119.6 (C-6), 116.3 (C-7), 70.5 (C-3), 55.7 (C-17), 54.6 (C-14), 46.3 (C-9), 42.8 (C-24), 42.8 (C-13), 40.8 (C-4), 40.5 (C-20), 39.1 (C-12), 38.4 (C-1), 37.0 (C-10), 33.1 (C-25), 32.0 (C-2), 28.3 (C-16), 23.0 (C-15), 21.1 (C-27), 21.1 (C-11), 20.0 (C-26), 19.7 (C-21), 17.6 (C-28), 16.3 (C-19), 12.1 (C-18)。以上数据与文献[26]基本一致,故鉴定为麦角甾醇。
化合物21 无色晶体,分子式C28H44O3。ESI-MS m/z: 451 [M + Na]+。1H NMR (500 MHz, CDCl3): δH 6.49 (1H, d, J = 8.5 Hz, H-6), 6.23 (1H, d, J = 8.5 Hz, H-7), 5.21 (1H, dd, J = 15.0, 7.5 Hz, H-23), 5.13 (1H, dd, J = 15.0, 8.5 Hz, H-22), 3.97 (1H, m, H-3), 2.10 (1H, dd, J = 6.0, 5.0 Hz, H-4a), 2.02 (1H, m, H-24), 1.94 (1H, m, H-2a), 1.94 (1H, m, H-1a), 1.91 (1H, m, H-4b), 1.87 (1H, m, H-20), 1.82 (1H, m, H-12), 1.69 (1H, m, H-1b), 1.60 (1H, m, H-11), 1.56 (1H, m, H-9), 1.49 (1H, m, H-12), 1.40 (1H, m, H-25), 1.34 (2H, m, H-16), 1.23 (1H, m, H-2b), 1.21 (2H, m, H-15), 1.18 (1H, m, H-14), 0.99 (3H, d, J = 6.5 Hz, CH3-28), 0.90 (3H, d, J = 6.5 Hz, CH3-21), 0.87 (3H, s, CH3-19), 0.83 (3H, s, CH3-18), 0.81 (3H, d, J = 6.5 Hz, CH3-26), 0.80 (3H, d, J = 6.5 Hz, CH3-27); 13C NMR (125 MHz, CDCl3): δC 135.4 (C-6), 135.2 (C-23), 132.3 (C-22), 130.8 (C-7), 82.2 (C-5), 79.4 (C-8), 66.5 (C-3), 56.2 (C-14), 51.7 (C-9), 51.1 (C-25), 44.6 (C-13), 42.8 (C-20), 39.8 (C-24), 39.3 (C-2), 37.0 (C-10), 36.9 (C-4), 34.7 (C-1), 33.1 (C-17), 30.1 (C-12), 28.7 (C-16), 23.4 (C-15), 20.9 (C-28), 20.6 (C-11), 20.0 (C-26), 19.7 (C-27), 18.2 (C-19), 17.6 (C-21), 12.9 (C-18)。以上数据与文献[27]基本一致,故鉴定为过氧麦角甾醇。
化合物22 白色固体,分子式C30H50O。ESI-MS m/z: 427 [M + H]+。1H NMR (600 MHz, CDCl3): δH 4.71 (1H, s, H-28b), 4.66 (1H, s, H-28a), 3.24 (1H, dd, J = 12.5, 4.5 Hz, H-3), 2.23 (1H, m, H-25), 2.16 (1H, m, H-23a), 2.02 (1H, m, H-1a), 1.85 (1H, m, H-23b), 1.74 (1H, m, H-2b), 1.56 (1H, m, H-2a), 1.40 (1H, m, H-20), 1.29 (1H, m, H-1b), 1.03 (1H, d, J = 3.6 Hz, CH3-27), 1.02 (3H, d, J = 3.6 Hz, CH3-26), 0.96 (3H, s, CH3-30), 0.95 (3H, s, CH3-19), 0.92 (3H, d, J = 6.3 Hz, CH3-21), 0.81 (3H, s, CH3-29), 0.59 (3H, s, CH3-18); 13C NMR (150 MHz, CDCl3): δC 156.9 (C-24), 135.8 (C-9), 127.9 (C-8), 105.9 (C-28), 79.0 (C-3), 54.7 (C-17), 51.9 (C-14), 50.2 (C-5), 42.1 (C-13), 38.9 (C-4), 37.0 (C-10), 37.0 (C-12), 36.3 (C-20), 35.7 (C-1), 34.6 (C-22), 33.8 (C-25), 31.1 (C-23), 28.8 (C-16), 28.4 (C-7), 28.0 (C-30), 27.9 (C-2), 23.8 (C-15), 22.5 (C-11), 22.0 (C-6), 22.0 (C-27), 21.9 (C-26), 19.8 (C-21), 18.7 (C-19), 15.4 (C-29), 11.3 (C-18)。以上数据与文献[28]基本一致,故鉴定为4, 4-dimethyl-5α-ergosta-8, 24 (28)-dien-3β-ol。
化合物23 白色固体,分子式C30H50O。ESI-MS m/z: 427 [M + H]+。1H NMR (600 MHz, CDCl3): δH 5.10 (1H, t, J = 7.2 Hz, H-24), 3.23 (1H, m, H-3), 1.69 (3H, s, CH3-27), 1.60 (3H, s, CH3-26), 1.00 (3H, s, CH3-29), 0.98 (3H, s, CH3-19), 0.91 (3H, d, J = 6.3 Hz, CH3-21), 0.87 (3H, s, CH3-28), 0.81 (3H, s, CH3-18), 0.69 (3H, s, CH3-30); 13C NMR (150 MHz, CDCl3): δC 134.4 (C-8), 134.4 (C-9), 130.9 (C-25), 125.2 (C-24), 79.0 (C-3), 50.4 (C-17), 50.4 (C-5), 49.8 (C-14), 44.5 (C-13), 38.9 (C-4), 37.0 (C-10), 36.4 (C-22), 36.3 (C-20), 35.6 (C-1), 31.0 (C-16), 30.8 (C-15), 30.8 (C-12) 28.0 (C-28), 27.8 (C-2), 26.5 (C-7), 25.7 (C-26), 24.9 (C-23), 24.2 (C-30), 21.0 (C-11), 19.1 (C-19), 18.6 (C-21), 18.2 (C-6), 17.6 (C-27), 15.7 (C-18), 15.4 (C-29)。以上数据与文献[29]基本一致,故鉴定为羊毛甾醇。
化合物24 无色油状,分子式C18H32O2。ESI-MS m/z: 279 [M – H]–。1H NMR (600 MHz, CDCl3): δH 5.35 (4H, m, H-9, 10, 12, 13), 2.77 (2H, t, J = 6.9 Hz, H-11), 2.34 (2H, t, J = 7.5 Hz, H-2), 2.05 (4H, t, J = 7.2 Hz, H-8, 14), 1.63 (2H, m, H-3), 1.34 (2H, m, H-17), 1.29 (10H, m, H-4~7, 15), 1.25 (2H, m, H-16), 0.89 (3H, t, J = 6.6 Hz, CH3-18); 13C NMR (150 MHz, CDCl3): δC 179.9 (C-1), 130.2 (C-10), 130.0 (C-13), 128.1 (C-9), 127.9 (C-12), 34.1 (C-2), 31.5 (C-16), 29.6 (C-7), 29.3 (C-15), 29.1 (C-5), 29.1 (C-6), 29.0 (C-4), 27.2 (C-8), 27.2 (C-14), 25.6 (C-11), 24.7 (C-3), 22.6 (C-17), 14.1 (C-18)。以上数据与文献[30]基本一致,故鉴定为亚油酸。
化合物25 无色油状,分子式C18H34O2。ESI-MS m/z: 281 [M – H]–。1H NMR (600 MHz, CDCl3): δH 5.34 (2H, m, H-9, 10), 2.34 (2H, t, J = 7.5 Hz, H-2), 2.01 (4H, t, J = 7.5 Hz, H-8, 11), 1.63 (2H, m, H-3), 1.32 (2H, m, H-17), 1.28 (16H, m, H-4~7, 12~15), 1.25 (2H, m, H-16), 0.88 (3H, t, J = 7.2 Hz, CH3-18); 13C NMR (150 MHz, CDCl3): δC 180.4 (C-1), 130.0 (C-9), 129.7 (C-10), 34.1 (C-2), 31.9 (C-16), 29.8 (C-13), 29.7 (C-14), 29.7 (C-15), 29.5 (C-12), 29.3 (C-4), 29.1 (C-5), 29.1 (C-6), 29.0 (C-7), 27.2 (C-8), 27.1 (C-11), 24.7 (C-3), 22.7 (C-17), 14.1 (C-18)。以上数据与文献[31]基本一致,故鉴定为油酸。
化合物26 黄色粉末,分子式C22H25N3O3。ESI-MS m/z: 402 [M + Na]+。1H NMR (500 MHz, CDCl3): δH 7.94 (1H, s, H-1), 7.42 (1H, d, J = 8.5 Hz, H-16), 6.84 (1H, d, J = 2.2 Hz, H-19), 6.81 (1H, dd, J = 8.5, 2.2 Hz, H-17), 5.98 (1H, dd, J = 9.5, 1.5 Hz, H-3), 4.90 (1H, dd, J = 9.5, 1.5 Hz, H-21), 4.17 (1H, ddd, J = 11.5, 5.0, 1.5 Hz, H-12), 4.09 (1H, ddd, J = 9.0, 7.0, 1.5 Hz, H-6), 3.82 (3H, s, 18-OCH3), 3.56 (2H, m, H-9), 3.51 (1H, dd, J = 16.0, 5.0 Hz, H-13a), 3.09 (1H, ddd, J = 16.0, 11.5, 1.5 Hz, H-13b), 2.40 (1H, m, H-7a), 2.22 (1H, m, H-7b), 2.05 (1H, m, H-8a), 1.93 (1H, m, H-8b), 1.99 (3H, s, CH3-24), 1.64 (3H, d, J = 1.5 Hz, CH3-23); 13C NMR (125 MHz, CDCl3): δC 169.6 (C-5), 165.8 (C-11), 156.5 (C-18), 137.0 (C-20), 134.0 (C-22), 132.2 (C-2), 124.2 (C-21), 120.8 (C-15), 118.9 (C-16), 109.5 (C-17), 106.2 (C-14), 95.3 (C-19), 59.3 (C-6), 56.8 (C-12), 55.8 (18-OCH3), 51.0 (C-3), 45.4 (C-9), 28.6 (C-7), 25.7 (C-23), 23.1 (C-8), 22.0 (C-13), 18.1 (C-24)。以上数据与文献[11]基本一致,故鉴定为烟曲霉毒素C。
化合物27 白色粉末,分子式C27H33N3O5。ESI-MS m/z: 981 [2M + Na]+。1H NMR (500 MHz, CDCl3): δH 7.84 (1H, d, J = 8.5 Hz, H-4), 6.79 (1H, dd, J = 8.5, 2.5 Hz, H-5), 6.69 (1H, d, J = 2.5 Hz, H-7), 5.97 (1H, d, J = 10.0, H-18), 5.76 (1H, dd, J = 2.5, 1.0 Hz, H-8), 5.03 (1H, t, J = 5.5 Hz, H-24), 4.70 (1H, dd, J = 12.0, 2.0 Hz, H-19), 4.54 (2H, d, J = 5.5 Hz, H-23), 4.45 (1H, dd, J = 10.0, 7.0 Hz, H-12), 3.84 (3H, s, 18-OCH3), 3.63 (2H, m, H-15), 2.47 (1H, m, H-13a), 2.10 (1H, m, H-13b), 2.08 (1H, m, H-14a), 1.99 (3H, s, CH3-21), 1.96 (1H, m, H-14b), 1.84 (3H, s, CH3-26), 1.69 (3H, s, CH3-27), 1.63 (3H, s, CH3-22); 13C NMR (125 MHz, CDCl3): δC 170.5 (C-11), 166.3 (C-5), 156.2 (C-18), 137.9 (C-20), 135.2 (C-27), 134.6 (C-23), 131.2 (C-2), 123.0 (C-26), 121.4 (C-16), 120.6 (C-15), 120.3 (C-22), 109.3 (C-17), 104.4 (C-14), 93.9 (C-19), 83.0 (C-12), 69.0 (C-13), 58.8 (C-6), 55.8 (18-OCH3), 49.1 (C-3), 45.3 (C-9), 41.8 (C-21), 29.0 (C-7), 25.7 (C-28), 25.6 (C-25), 22.6 (C-8), 18.4 (C-29), 18.2 (C-24)。以上数据与文献[32]基本一致,故鉴定为烟曲霉毒素B。
化合物28 白色粉末,分子式C27H33N3O7。ESI-MS m/z: 534 [M + Na]+。1H NMR (500 MHz, CDCl3): δH 7.90 (1H, d, J = 8.0 Hz, H-16), 6.83 (1H, dd, J = 8.0, 2.5 Hz, H-17), 6.64 (1H, d, J = 8.0 Hz, H-21), 6.60 (1H, d, J = 2.5 Hz, H-19), 6.05 (1H, d, J = 10.5 Hz, H-3), 5.66 (1H, d, J = 2.5 Hz, H-13), 5.04 (1H, dt, J = 8.0, 1.5 Hz, H-22), 4.48 (1H, dd, J = 10.0, 7.0 Hz, H-6), 3.84 (3H, s, 18-OCH3), 3.63 (2H, m, H-9), 2.51 (2H, m, H-7), 2.11 (2H, m, H-8), 2.03 (2H, m, H-26), 2.00 (3H, d, J = 1.5 Hz, CH3-25), 1.97 (1H, m, H-8), 1.74 (3H, d, J = 1.5 Hz, CH3-24), 1.72 (3H, s, CH3-29), 1.67 (1H, m, H-26), 1.01 (3H, s, CH3-28); 13C NMR (125 MHz, CDCl3): δC 170.7 (C-11), 166.2 (C-5), 156.4 (C-18), 143.2 (C-23), 136.2 (C-20), 131.6 (C-2), 121.7 (C-16), 121.0 (C-15), 118.5 (C-22), 109.4 (C-17), 105.5 (C-14), 93.9 (C-19), 85.8 (C-21), 82.5 (C-12), 82.1 (C-27), 68.7 (C-13), 58.7 (C-6), 55.8 (18-OCH3), 51.2 (C-9), 48.9 (C-3), 45.3 (C-26), 29.1 (C-7), 27.1 (C-28), 25.7 (C-29), 24.2 (C-25), 22.6 (C-8), 18.8 (C-24)。以上数据与文献[33]基本一致,故鉴定为震颤真菌毒素。
化合物29 淡黄色粉末,分子式C22H25NO8。ESI-MS m/z: 454 [M + Na]+。1H NMR (600 MHz, CDCl3): δH 8.31 (2H, m, H-19, 23), 7.65 (1H, t, J = 7.5 Hz, H-21), 7.49 (2H, t, J = 7.5 Hz, H-20, 22), 5.62 (1H, dt, J = 11.0, 7.5 Hz, H-13), 5.30 (1H, dd, J = 11.0, 9.0 Hz, H-12), 4.75 (1H, s, H-9), 4.69 (1H, d, J = 11.0 Hz, H-11), 4.59 (1H, s, H-10), 3.42 (3H, s, 8-OCH3), 2.18 (1H, m, H-14), 2.11 (1H, m, H-14), 1.69 (3H, s, CH3-16), 0.99 (3H, t, J = 7.5 Hz, CH3-15); 13C NMR (150 MHz, CDCl3): δC 196.4 (C-4), 195.0 (C-17), 185.8 (C-6), 166.6 (C-2), 137.0 (C-13), 134.8 (C-21), 132.3 (C-18), 130.7 (C-19), 130.7 (C-23), 128.7 (C-20), 128.7 (C-22), 125.4 (C-12), 113.5 (C-3), 92.7 (C-5), 90.3 (C-8), 73.1 (C-9), 70.7 (C-10), 70.6 (C-11), 51.8 (8-OCH3), 21.4 (C-14), 14.1 (C-15), 6.0 (C-16)。以上数据与文献[34]基本一致,故鉴定为pseurotin A。
化合物30 白色粉末,分子式C24H21N5O4。ESI-MS m/z: 466 [M + H]+。1H NMR (500 MHz, CDCl3): δH 8.35 (1H, dd, J = 8.0, 1.5 Hz, H-10), 7.84 (1H, ddd, J = 8.0, 7.0, 1.5 Hz, H-8), 7.74 (1H, q, J = 8.0 Hz, H-7), 7.67 (1H, d, J = 8.0 Hz, H-9), 7.36 (1H, d, J = 8.0 Hz, H-24), 7.31 (1H, dd, J = 8.0, 7.0 Hz, H-27), 7.27 (1H, d, J = 8.0 Hz, H-25), 7.18 (1H, td, J = 8.0, 1.0 Hz, H-26), 5.72 (1H, dd, J = 7.5, 2.0 Hz, H-14), 5.34 (1H, d, J = 7.5 Hz, H-18), 3.71 (1H, q, J = 7.0 Hz, H-20), 2.72 (1H, d, J = 8.0 Hz, H-15b), 2.13 (1H, d, J = 15.0 Hz, H-15a), 2.05 (3H, s, H-16), 1.04 (1H, d, J = 7.0 Hz, 19-NH), 1.03 (3H, d, J = 7.0 Hz, CH3-29); 13C NMR (125 MHz, CDCl3): δC 170.9 (C-1), 170.8 (C-21), 159.5 (C-12), 150.4 (C-4), 146.3 (C-4), 138.4 (C-28), 135.7 (C-23), 135.0 (C-8), 130.3 (C-25), 128.6 (C-9), 128.5 (C-7), 127.0 (C-10), 125.2 (C-21), 124.9 (C-27), 121.4 (C-11), 115.5 (C-24), 87.1 (C-17), 87.1 (C-18), 84.2 (C-3), 58.6 (C-20), 51.4 (C-14), 31.4 (C-15), 24.5 (C-16), 18.8 (C-29)。以上数据与文献[24]基本一致,故鉴定为fumiquinazoline C。
化合物31 橙黄色粉末,分子式C16H12O5。ESI-MS m/z: 283 [M – H]–。1H NMR (600 MHz, Acetone-d6): δH 13.32 (1H, s, 1-OH), 7.49 (1H, d, J = 2.1 Hz, H-4), 7.34 (1H, d, J = 2.4 Hz, H-5), 7.09 (1H, s, H-2), 6.93 (1H, d, J = 2.4 Hz, H-7), 3.98 (3H, s, 8-OCH3), 2.43 (3H, s, CH3-3); 13C NMR (150 MHz, Acetone-d6): δC 187.8 (C-9), 183.2 (C-10), 165.0 (C-8), 164.8 (C-6), 163.5 (C-1), 147.6 (C-3), 138.4 (C-10′), 133.4 (C-4′), 125.0 (C-2), 119.9 (C-4), 114.5 (C-9′), 112.6 (C-8′), 107.6 (C-5), 105.7 (C-7), 56.7 (8-OCH3), 21.8 (C-3)。以上数据与文献[35]基本一致, 故鉴定为questin。
2 化合物活性评价分离鉴定的化合物大部分量都较少,主要类型为二酮哌嗪类衍生物(DKP)及异螺环-γ内酰胺,这两类化合物均具有广泛的生物活性[6–7, 36],故主要对发酵液和菌丝体中均分离得到的这两类化合物进行活性评价。
2.1 乙酰胆碱酯酶抑制活性评价采用改良的Ellman光度法[37]对化合物26~29进行乙酰胆碱酯酶抑制活性测定。化合物用DMSO和水稀释成1 mmol/L工作液,保证不同浓度的化合物溶液中DMSO浓度相同(均为2%),化合物终浓度为50 μmol/L。阳性对照为他克林,终浓度为0.333 μmol/L;阴性对照为2% DMSO溶剂。在96孔酶标板中加入磷酸盐缓冲液(pH 8.0)、测试化合物(50 μmol/L)和乙酰胆碱酯酶(0.02 U/mL)的反应混合物(共200 μL)孵育20 min (37 ℃)。然后,分别加入40 μL含有DTNB (0.625 mmol/L)和碘化硫代乙酰胆碱(0.625 mmol/L)的溶液来启动反应,用于AChE抑制活性的测定。加入显色剂和底物后1 h内,每30 s检测1次405 nm波长下的吸光值。每个样品重复3次。抑制率(%)=(E-S/E)×100%,式中,E为未加测试化合物的酶活性,S为加入测试化合物的酶活性。结果表明,化合物26~29均无明显乙酰胆碱酯酶抑制活性(抑制率 < 10%)。
2.2 烟草黑胫病菌抑制活性评价按照GB/T 38480—2020标准,采用菌丝生长速率法对化合物26~29进行烟草黑胫病菌抑制活性测定。化合物和阳性对照(潮霉素B)溶于DMSO配成2 mg/mL母液,在无菌操作条件下,将预先融化的灭菌培养基定量加入无菌三角瓶中(每个10 mL),冷却后分别加入0.5 mL药剂母液,充分摇匀;然后均匀倒入培养皿中,分别制成浓度为100 μg/mL的含药平板。实验设置阴性对照(DMSO)和空白对照。将培养好的植物病原菌,在无菌条件下用灭菌打孔器自菌落边缘切取生长一致的菌饼,用接种针将菌饼接种于含药平板中央(有菌丝面与培养基接合), 将培养皿置于36 ℃的恒温培养箱,每天进行观察菌丝生长情况。用游标卡尺测量菌落直径,单位为mm。每个菌落用十字交叉法垂直测量直径各1次,取其平均值,计算菌丝生长抑制率(%)=[(对照菌落直径-处理菌落直径)/对照菌落直径]×100%。结果表明,化合物26和27对烟草黑胫病菌具有微弱的抑制作用,抑菌率分别为19.64%和17.86% (图 4)。
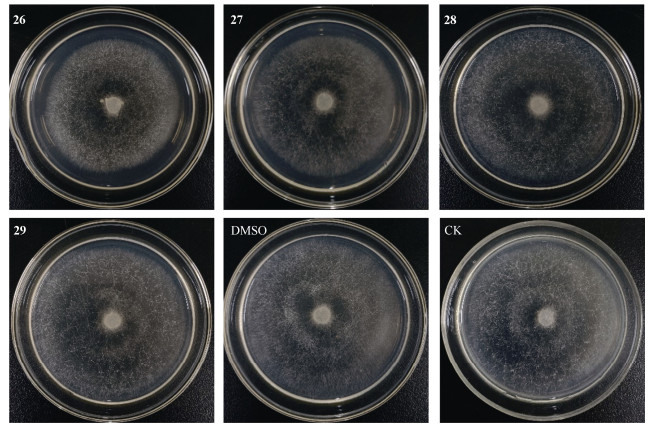
|
图 4 烟草黑胫病菌抑制活性 Fig. 4 Tobacco black shank inhibition activity |
本研究从滇重楼内生真菌A. fumigatus的发酵液和菌丝体中共分离得到31个化合物,主要为生物碱类化合物(19个),其中化合物15和23为首次从烟曲霉属真菌中分离得到。化合物1~16仅从发酵液中分离得到;化合物17~25仅从菌丝体中分离得到;化合物26~31在发酵液和菌丝体中均分离得到。化合物20 (麦角甾醇)为真菌细胞膜的重要组成成分, 化合物21、22和23为其前体,因而化合物20~23仅在菌丝体中分离得到,这表明菌丝体和发酵液中的代谢产物结构类型并无明显差异。值得注意的是,DKP及异螺环-γ内酰胺是发酵液和菌丝体中共有的结构类型,推测可能是由于此类成分在培养过程中由菌丝体产生随后分布到发酵液中。与此同时,近20年对内生真菌A. fumigatus的研究表明, 大部分研究分离的次生代谢产物中都含有DKP及异螺环-γ内酰胺[4–9, 21, 36, 38–49];另一方面,以往研究所使用的培养基主要为固体大米培养基、液体察氏培养基、液体PDB培养基等,尽管培养条件不同,但都得到了基本一致的结果[6, 49];故此内生真菌的培养条件不是影响其次生代谢产物的主要因素。
本研究对化合物26~29进行了乙酰胆碱酯酶抑制活性和烟草黑胫病菌抑制活性的评价,结果表明这4个化合物均无明显乙酰胆碱酯酶抑制活性(抑制率 < 10%),但化合物26和27对烟草黑胫病菌具有微弱的抑制作用,抑菌率分别为19.64%和17.86%。本研究丰富了A. fumigatus次生代谢产物的化学结构多样性,为进一步从生物间化学行为的角度探究滇重楼与其内生真菌相互作用规律和机制提供基础。
| [1] |
TORRESMENDOZA D, ORTEGA H E, CUBILLA-RIOS L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications[J]. J Fungi, 2020, 6(2): 58. DOI:10.3390/jof6020058 |
| [2] |
Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia[M]. Beijing: China Medical Science Press, 2020: 271. 国家药典委员会. 中华人民共和国药典(一部)[M]. 北京: 中国医药科技出版社, 2020: 271. |
| [3] |
FRISVAD J C, RANK C, NIELSEN K F, et al. Metabolomics of Aspergillus fumigatus[J]. Med Mycol, 2009, 47(S1): S53-S71. DOI:10.1080/13693780802307720 |
| [4] |
LIU S, DAI H F, KONUKLUGIL B, et al. Phenolic bisabolanes from the sponge-derived fungus Aspergillus sp.[J]. Phytochem Lett, 2016, 18: 187-191. DOI:10.1016/j.phytol.2016.10.015 |
| [5] |
LUO S L, LI G H, LIU F F, et al. A new sesquiterpene from endophytic fungus Aspergillus sp.[J]. Nat Prod Res, 2012, 26(14): 1334-1338. DOI:10.1080/14786419.2011.583242 |
| [6] |
MARTÍNEZ-LUIS S, CHERIGO L, ARNOLD E, et al. Antiparasitic and anticancer constituents of the endophytic fungus Aspergillus sp. strain F1544[J]. Nat Prod Commun, 2012, 7(2): 165-168. DOI:10.1177/1934578X1200700207 |
| [7] |
LI X J, ZHANG Q, ZHANG A L, et al. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities[J]. J Agric Food Chem, 2012, 60(13): 3424-3431. DOI:10.1021/jf300146n |
| [8] |
LUO G Y, LANG J J, SHE Z G, et al. Nitrogen-containing compounds from mangrove-derived fungus Aspergillus sp. 87[J]. Nat Prod Commun, 2020, 15(4): 1-4. DOI:10.1177/1934578X20915314 |
| [9] |
KONG F D, HUANG X L, MA Q Y, et al. Helvolic acid derivatives with antibacterial activities against Streptococcus agalactiae from the marine-derived fungus Aspergillus fumigatus HNMF0047[J]. J Nat Prod, 2018, 81(8): 1869-1876. DOI:10.1021/acs.jnatprod.8b00382 |
| [10] |
CHEN J Y. The endophytic fungus RPF8 from Paris polyphylla Smith and its antitumor active aomponents research[D]. Hangzhou: Zhejiang Chinese Medical University, 2013: 27–28. 陈金印. 华重楼内生真菌RPF8发酵产物抗肿瘤作用研究[D]. 杭州: 浙江中医药大学, 2013: 27–28. |
| [11] |
CUI C B, KAKEYA H, OSADA H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus: Ⅱ. Physico-chemical properties and structures[J]. J Antibiot, 1996, 49(6): 534-540. DOI:10.7164/antibiotics.49.534 |
| [12] |
ABRAHAM W R, ARFMANN H A. 12, 13-dihydroxy-fumitremorgin C from Aspergillus fumigatus[J]. Phytochemistry, 1990, 29(3): 1025-1026. DOI:10.1016/0031-9422(90)80080-Z |
| [13] |
CUI C B, KAKEYA H, OSADA H. Novel mammalian cell cycle inhibitors, cyclotroprostatins A–D, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase[J]. Tetrahedron, 1997, 53(1): 59-72. DOI:10.1016/S0040-4020(96)00978-7 |
| [14] |
ZHANG M, WANG W L, FANG Y C, et al. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi[J]. J Nat Prod, 2008, 71(6): 985-989. DOI:10.1021/np700737g |
| [15] |
WINK J, GRABLEY S, GAREIS M, et al. Pseurotin F1/F2, neue metabolite aus Aspergillus fumigatus, verfahren zu ihrer herstellung und ihre verwendung als apomorphin antagonisten [P]. 1993.
|
| [16] |
REN H, LIU R, CHEN L, et al. Two new hetero-spirocyclic γ-lactam derivatives from marine sediment-derived fungus Aspergillus sydowi D2–6[J]. Arch Pharm Res, 2010, 33(4): 499-502. DOI:10.1007/s12272-010-0401-4 |
| [17] |
BREITENSTEIN W, CHEXAL K K, MOHR P, et al. Pseurotin B, C, D, and E: Further new metabolites of Pseudeurotium ovalis STOLK[J]. Helv Chim Acta, 1981, 64(2): 379-388. DOI:10.1002/hlca.19810640203 |
| [18] |
SHI Y S, ZHANG Y, CHEN X Z, et al. Metabolites produced by the endophytic fungus Aspergillus fumigatus from the stem of Erythro-phloeum fordii Oliv[J]. Molecules, 2015, 20(6): 10793-10799. DOI:10.3390/molecules200610793 |
| [19] |
LAN W J, FU S J, XU M Y, et al. Five new cytotoxic metabolites from the marine fungus Neosartorya pseudofischeri[J]. Mar Drugs, 2016, 14(1): 18. DOI:10.3390/md14010018 |
| [20] |
CHU M, MIERZWA R, HE L, et al. Structure of sch 528647: A new antitumor antibiotic related to fumagillin[J]. J Antibiot, 2001, 54(12): 1096-1099. DOI:10.7164/antibiotics.54.1096 |
| [21] |
LI S, CHEN J F, QIN L L, et al. Two new sesquiterpenes produced by the endophytic fungus Aspergillus fumigatus from Ligusticum wallichii[J]. J Asian Nat Prod Res, 2020, 22(2): 138-143. DOI:10.1080/10286020.2018.1540606 |
| [22] |
ZHANG P, BAO B Q, DANG H T, et al. Anti-inflammatory sesquiterpenoids from a sponge-derived fungus Acremonium sp.[J]. J Nat Prod, 2009, 72(2): 270-275. DOI:10.1021/np8006793 |
| [23] |
OHTANI K, FUJIOKA S, SHIMADA A, et al. Nematicidal activities of 4-hydroxyphenylacetic acid and oidiolactone D produced by the fungus Oidiodendron sp.[J]. Z Naturforsch C J Biosci, 2011, 66(1/2): 31-34. DOI:10.1515/znc-2011-1-205 |
| [24] |
TAKAHASHI C, MATSUSHITA T, DOI M, et al. Fumiquinazolines A–G, novel metabolites of a fungus separated from a Pseudolabrus marine fish[J]. J Chem Soc, Perkin Trans, 1995, 1(18): 2345-2353. DOI:10.1039/P19950002345 |
| [25] |
LEE S Y, KINOSHITA H, IHARA F, et al. Identification of novel derivative of helvolic acid from Metarhizium anisopliae grown in medium with insect component[J]. J Biosci Bioeng, 2008, 105(5): 476-480. DOI:10.1263/jbb.105.476 |
| [26] |
LIANGSAKUL J, SRISURICHAN S, PORNPAKAKUL S. Anthraqui-none-steroids, evanthrasterol A and B, and a meroterpenoid, emericellic acid, from endophytic fungus, Emericella variecolor[J]. Steroids, 2016, 106: 78-85. DOI:10.1016/j.steroids.2015.12.012 |
| [27] |
KWON H C, ZEE S D, CHO S Y, et al. Cytotoxic ergosterols from Paecilomyces sp. J300[J]. Arch Pharm Res, 2002, 25(6): 851-855. DOI:10.1007/BF02977003 |
| [28] |
FATTORUSSO E, GIOVANNITTI B, LANZOTTI V, et al. 4, 4-Dimethyl-5α-ergosta-8, 24(28)-dien-3β-ol from the fungus Marasmius oreades[J]. Steroids, 1992, 57(3): 119-121. DOI:10.1016/0039-128X(92)90069-L |
| [29] |
HU C, CHEN F, ZHENG X H, et al. Chemical compounds from the fermented broth of actinomycete strain Streptomyces antibioticus H12-15[J]. Nat Prod Res Dev, 2017, 29(3): 404-409. 胡辰, 陈芳, 郑新恒, 等. 链霉属放线菌Streptomyces antibioticus H12-15发酵物的化学成分研究[J]. 天然产物研究与开发, 2017, 29(3): 404-409. DOI:10.16333/j.1001-6880.2017.3.007 |
| [30] |
TANG X, PEI G, ZHOU Z Y, et al. Chemical constituents from roots of Achyranthes bidentata[J]. J Trop Subtrop Bot, 2013, 21(1): 57-62. 唐鑫, 裴刚, 周忠玉, 等. 牛膝根化学成分研究[J]. 热带亚热带植物学报, 2013, 21(1): 57-62. DOI:10.3969/j.issn.1005-3395.2013.01.008 |
| [31] |
HE Y, LEI D Y, YANG Q Q, et al. Xanthium orientale subsp. italicum (Moretti) Greuter: Bioassay-guided isolation and its chemical basis of antitumor cytotoxicity[J]. Nat Prod Res, 2021, 35(14): 2433-2437. DOI:10.1080/14786419.2019.1672686 |
| [32] |
YAMAZAKI M, FUJIMOTO H, KAWASAKI T. Chemistry of tremorogenic metabolites: Ⅰ. Fumitremorgin A from Aspergillus fumigatus[J]. Chem Pharm Bull, 1980, 28(1): 245-254. DOI:10.1248/cpb.28.245 |
| [33] |
FAYOS J, LOKENSGARD D, CLARDY J, et al. Structure of verruculogen, a tremor producing peroxide from Penicillium verruculosum[J]. J Am Chem Soc, 1974, 96(21): 6785-6787. DOI:10.1021/ja00828a054 |
| [34] |
BLOCH P, TAMM C. Isolation and structure of pseurotin A, a microbial metabolite of Pseudeurotium ovalis STOLK with an unusual heterospirocyclic system[J]. Helv Chim Acta, 1981, 64(1): 304-315. DOI:10.1002/hlca.19810640131 |
| [35] |
FUJIMOTO H, FUJIMAKI T, OKUYAMA E, et al. Immunomodulatory constituents from an ascomycete, Microascus tardifaciens[J]. Chem Pharm Bull, 1999, 47(10): 1426-1432. DOI:10.1248/cpb.47.1426 |
| [36] |
ZHANG H C, MA Y M, LIU R, et al. Endophytic fungus Aspergillus tamarii from Ficus carica L., a new source of indolyl diketopiperazines[J]. Biochem Syst Ecol, 2012, 45: 31-33. DOI:10.1016/j.bse.2012.07.020 |
| [37] |
ELLMAN G L, COURTNEY K D, ANDRES V JR, et al. A new and rapid colorimetric determination of acetylcholinesterase activity[J]. Biochem Pharmacol, 1961, 7(2): 88-95. DOI:10.1016/0006-2952(61)90145-9 |
| [38] |
WANG F Z, FANG Y C, ZHU T J, et al. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus[J]. Tetrahedron, 2008, 64(34): 7986-7991. DOI:10.1016/j.tet.2008.06.013 |
| [39] |
ZOU R J, WEI C W, ZHANG X X, et al. Alkaloids from endophytic fungus Aspergillus fumigatus HQD24 isolated from the Chinese mangrove plant Rhizophora mucronata[J]. Nat Prod Res, 2022, 36(19): 5069-5073. DOI:10.1080/14786419.2021.1916017 |
| [40] |
GE H M, YU Z G, ZHANG J, et al. Bioactive alkaloids from endophytic Aspergillus fumigatus[J]. J Nat Prod, 2009, 72(4): 753-755. DOI:10.1021/np800700e |
| [41] |
XU J, SONG Y C, GUO Y, et al. Fumigaclavines D–H, new ergot alkaloids from endophytic Aspergillus fumigatus[J]. Planta Med, 2014, 80(13): 1131-1137. DOI:10.1055/s-0034-1382958 |
| [42] |
LIU L, NI H F, QIU X, et al. Cytotoxic tetracyclic triterpenes from the endophytic fungus Aspergillus fumigatus of Cleidion brevipetiolatum[J]. Phytochem Lett, 2021, 44: 87-89. DOI:10.1016/j.phytol.2021.06.005 |
| [43] |
GUO D L, LI X H, FENG D, et al. Novel polyketides produced by the endophytic fungus Aspergillus fumigatus from Cordyceps sinensis[J]. Molecules, 2018, 23(7): 1709. DOI:10.3390/molecules23071709 |
| [44] |
JIANG Y, JIANG C X, ZHOU Q, et al. A new alkaloid from the endophytic fungus of Crocus sativus L., Aspergillus fumigatus Y0107[J]. Rec Nat Prod, 2022, 16(5): 463-470. DOI:10.25135/rnp.307.2110.2235 |
| [45] |
LIANG Z Z, ZHANG T T, ZHANG X Q, et al. An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus[J]. Molecules, 2015, 20(1): 1424-1433. DOI:10.3390/molecules20011424 |
| [46] |
LIU J Y, SONG Y C, ZHANG Z, et al. Aspergillus fumigatus CY018, an endophytic fungus in Cynodon dactylon as a versatile producer of new and bioactive metabolites[J]. J Biotechnol, 2004, 114(3): 279-287. DOI:10.1016/j.jbiotec.2004.07.008 |
| [47] |
XIE F, LI X B, ZHOU J C, et al. Secondary metabolites from Aspergillus fumigatus, an endophytic fungus from the liverwort Heteroscyphus tener (Steph.) Schiffn[J]. Chem Biodiv, 2015, 12(9): 1313-1321. DOI:10.1002/cbdv.201400317 |
| [48] |
LHAMO S, WANG X B, LI T X, et al. Three unusual indole diketopiperazine alkaloids from a terrestrial-derived endophytic fungus, Aspergillus sp.[J]. Tetrahedron Lett, 2015, 56(21): 2823-2826. DOI:10.1016/j.tetlet.2015.04.058 |
| [49] |
ZHANG Q, WANG S Q, TANG H Y, et al. Potential allelopathic indole diketopiperazines produced by the plant endophytic Aspergillus fumigatus using the one strain-many compounds method[J]. J Agric Food Chem, 2013, 61(47): 11447-11452. DOI:10.1021/jf403200g |
 2024, Vol. 32
2024, Vol. 32


