2. 吉林农业大学食品科学与工程学院, 长春 130118;
3. 华南理工大学食品科学与工程学院, 广州 510641
2. College of Food Science and Engineering, Jilin Agricultural University, Changchun 130118, China;
3. College of Food Science and Engineering, South China University of Technology, Guangzhou 510641, China
无花果亚属(Ficus subgenus)是榕属(Ficus genus)中最大的开花植物属,包含700余种植物[1]。原产于西南亚和地中海地区,现已在热带、亚热带及温带地区广泛种植[2]。无花果亚属植物具有重要的食用和药用价值[3–4], 其果实在很多国家可食用, 根和叶则被应用于传统医药。在越南,地果(F. tikoua)广泛用于治疗水肿、痢疾、脓疱等病症[5],因其提取物具有显著的抗菌活性[6],常被用来提取精油。在印度,对叶榕(F. hispida)常用于治疗皮肤、呼吸系统和泌尿系统等的疾病[7]。作为无花果亚属中最具商业价值的水果,无花果(F. carica)既是口感甜美的水果食品,同时也具有祛痰、疏肝、消炎、抗癌功效并用于治疗肝脾疾病等[8–9]。查阅近年来国内外对无花果亚属植物化学成分和生物活性的报道, 该亚属植物富含类黄酮[10]、三萜、香豆素等活性成分[11–12]。其中,一些香豆素类成分,如马豆素、补骨脂素、香柠檬烯等可减少氧化应激,防止胰岛β细胞损伤,表现出降血糖活性[13]。部分萜类成分, 如鲍尔烯醇、羽扇豆醇、齐墩果酸等具有抗氧化、抗炎、保肝等功效[11]。此外,异戊烯基黄酮类物质是该类植物的特征性物质,已成为近年来学者们关注的热点,具有重要的药用价值。本文对无花果亚属中异戊烯基黄酮类化合物进行综述。
1 结构类型无花果亚属植物中,已报道47个异戊烯基黄酮类化合物。异戊烯基主要存在形式有13种(图 1),其中二甲基烯丙基是主要的结构类型。取代位点发生在类黄酮A环或B环,以A-异戊烯基化为主, 最常见的取代位点为A-6位和A-8位;部分取代发生在B环上,主要取代位点为B-2ʹ和B-4ʹ处。除异戊烯基取代外,黄酮母核上通常还连有羟基和甲氧基。无花果亚属植物异戊烯基黄酮的类黄酮骨架类型主要以异黄酮为主,还包含少量的黄烷酮、黄酮、查尔酮(图 2, 3)。
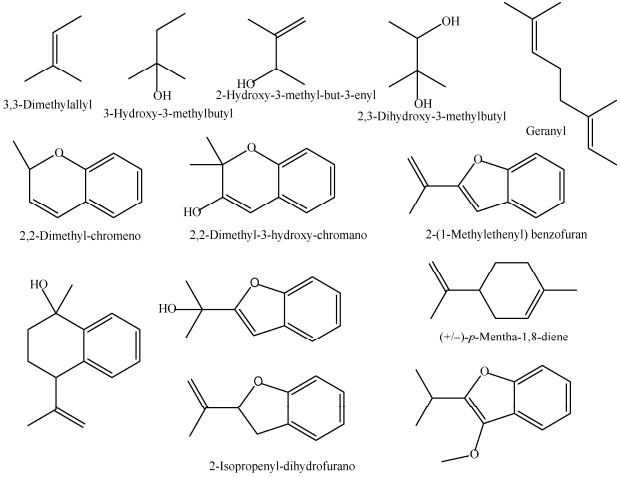
|
图 1 异戊烯基结构 Fig. 1 Structures of prenylation |
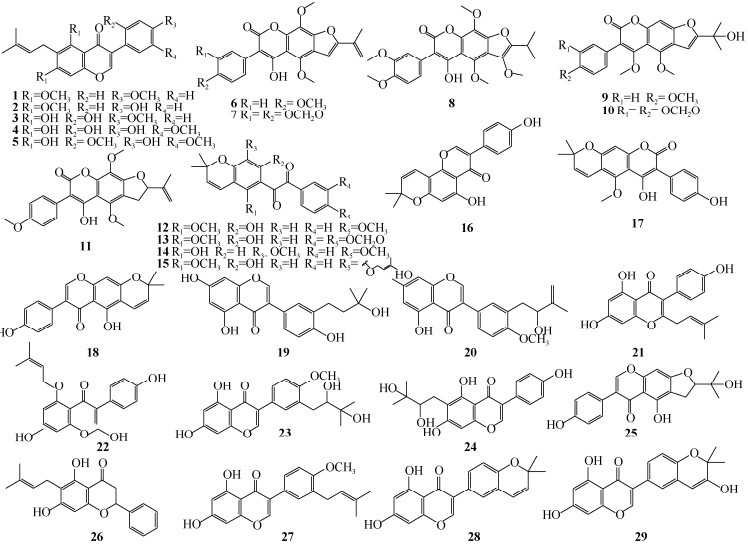
|
图 2 无花果亚属植物异戊烯基类黄酮成分结构(1~29) Fig. 2 Structures of prenylated flavonoids in Ficus subgenus (1-29) |
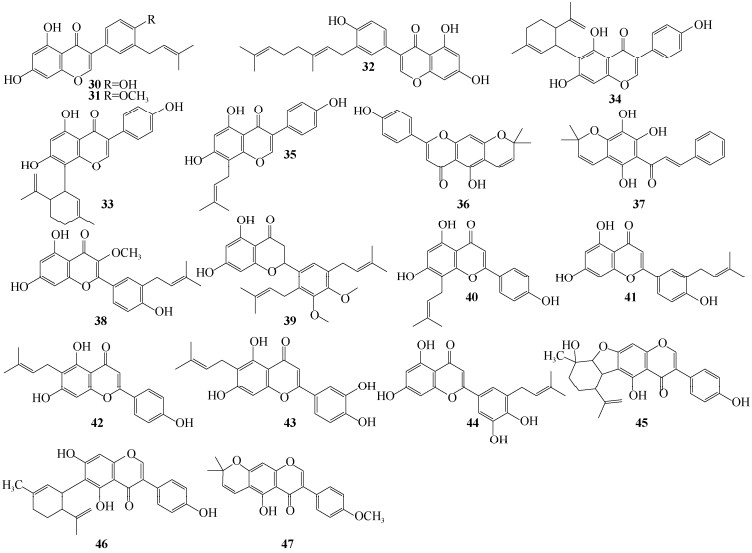
|
图 3 无花果亚属植物异戊烯基类黄酮成分结构(30~47) Fig. 3 Structures of prenylated flavonoids in Ficus subgenus (30-47) |
黄酮类化合物是重要天然活性物质,安全性好,具有抗肿瘤、抗病毒、抗炎、抗菌等活性。已有化学及生物活性研究结果表明,大多数情况下, 异戊烯基基团的引入会增加类黄酮的亲脂性,改善对生物膜的亲和性,显著提高生物活性[14–15]。表 1列出了已报道的无花果亚属植物异戊烯基类黄酮的生物活性。
| 表 1 无花果亚属植物异戊烯基类黄酮 Table 1 Prenylated flavonoids in Ficus subgenus |
异戊烯基异黄酮是无花果异戊烯基类黄酮的主要结构形式,在结构上与雌激素相似[16],可以与雌激素α、β受体结合,并具有选择性。通过发挥雌激素受体调节活性,异戊烯基异黄酮可缓解更年期症状,保护骨骼,预防乳腺癌[17]等疾病。在已经报道的47种异戊烯基类黄酮中,37种为异戊烯基异黄酮。因此,无花果亚属植物是丰富的异戊烯基异黄酮资源,可用于功能食品或药物开发。
2.2 抗氧化氧化损伤是引发癌症、心血管疾病、动脉粥样硬化等多种慢性病的重要因素[18],常常伴随炎症的产生。通过抑制或延迟氧化反应来预防和治疗疾病十分重要[19]。作为膳食抗氧化剂,食源性黄酮类成分在减缓氧化损伤中具有重要作用。已有研究表明[5, 7, 11, 20–21],无花果中大部分异戊烯基类黄酮成分(化合物17、18、21、22、29、35、37、42、45、46、47)表现出优良的清除DPPH自由基活性。抗氧化活性强弱和异戊烯基类黄酮的母核结构、官能团排列、酚羟基数目直接相关。B环羟基与2, 3-烯基的存在是影响抗氧化活性的重要因素[22]。此外, Popoola等[23]的研究表明,化合物26还可以抑制酪氨酸酶活性,减少体内自由基的积累,进而预防皮肤衰老。
2.3 预防炎症炎症,通常是机体对抗外界感染而产生的防御性免疫反应[24]。炎症失衡可引发哮喘、糖尿病等多种急、慢性并发症[15]。在无花果异戊烯基类黄酮中,17种成分通过调节促炎分子合成和炎症相关细胞因子分泌而表现出抗炎活性。其中,化合物1~15通过抑制NO的产生而缓解炎症反应[25]。化合物47对抑制单核细胞/巨噬细胞活化具有重要作用[26]。在这些具有抗炎活性的化合物中,9~11、13表现出强抗炎活性(IC50值< 2 μmol/L)[25]。这几个物质的类黄酮骨架结构与异戊烯基结构特征是影响其抗炎活性的关键因素。
2.4 预防癌症抗炎治疗是治疗早期肿瘤进展和恶性转化的一种有效的治疗方法。无花果异戊烯基类黄酮在发抗炎活性的同时,也具有抗癌细胞增殖作用[27]。
此外,异戊烯基类黄酮通过调节不同分子靶点发挥癌症预防作用。主要机制包括诱导细胞凋亡、抑制血管生成、调控遗传因子、转录因子等[28]。如化合物16可通过诱导细胞中ROS和ERK抑制癌细胞增殖[29];化合物44可抑制A549和NCI-292细胞的增殖[30]。化合物18通过诱导肺细胞、食管鳞状细胞、肾细胞凋亡抗癌[21];化合物20对TPA诱导的Raji细胞中EBV-EA活化的抑制作用明显[31]; 化合物35可抑制前列腺癌细胞系(DU-145)和静脉内皮细胞系(HUVEC)实现抗肿瘤和抗血管生成作用[32];化合物42通过MAPK和AKT信号传导途径诱导HeLa细胞凋亡预防癌症[33]。
2.5 抗菌活性不同于常规药物,植物类黄酮在发挥抗菌活性的同时具有较好的安全性,可以选择性地靶向细菌细胞,抑制毒性因子和微生物威胁的同时,不会引发抗生素耐药性等问题[53],已成为目前最受欢迎的生物抗菌剂之一。无花果异戊烯基类黄酮物质中, 化合物18和30对金黄色葡萄球菌,大肠杆菌、李斯特菌具有抑制作用[30–31]。化合物17可抑制结核分岐杆菌[38], 表现出抗结核作用,可用于开发肺部感染的抗菌药物。Lopes等[28, 33]的研究表明,化合物2和4具有明显抗真菌活性,可能是由于羟基化程度的增加导致对微生物的抑制能力提升。此外,化合物21和22也表现出抗菌活性[11],其抗菌机理还需进一步研究。
2.6 其他活性Sakat等[7]报道,化合物31~34和38可通过抑制α-葡萄糖苷酶活性预防糖尿病。与目前在T2D的治疗中使用的α-葡萄糖苷酶抑制剂(如阿卡波糖)相比,不产生胃肠道副作用,有希望成为新型和更安全的治疗药物。Jung-Hae等[43]报道,化合物28可通过抑制血小板来预防血栓。Matsuda等[42]的研究表明,化合物24可抑制d-半乳糖胺诱导的小鼠原代肝细胞的细胞毒性,具有保肝作用。Williams等[35]的研究表明,化合物3、25和33可通过抑制BACE1活性预防阿尔兹海默症。
3 展望因为富含结构多样、活性突出的异戊烯基类黄酮物质,无花果亚属植物已成为功能食品、天然药物领域的重要原料来源。在未来研究中,仍需加强对无花果亚属植物的化学成分发掘,深入阐明其生物活性作用机理以及构效关系。相关研究结果对于该亚属植物的综合开发与利用具有重要意义。
| [1] |
RØNSTED N, WEIBLEN G D, CLEMENT W L, et al. Reconstructing the phylogeny of figs (Ficus, Moraceae) to reveal the history of the fig pollination mutualism[J]. Symbiosis, 2008, 45(1): 45-55. |
| [2] |
RAHMANI A H, ALDEBASI Y H. Ficus carica and its constituents role in management of diseases[J]. Asian J Pharm Clin Res, 2017, 10(6): 49-53. DOI:10.22159/ajpcr.2017.v10i6.17832 |
| [3] |
AYOUB L, HASSAN F, HAMID S, et al. Phytochemical screening, antioxidant activity and inhibitory potential of Ficus carica and Olea europaea leaves[J]. Bioinformation, 2019, 15(3): 226-232. DOI:10.6026/97320630015226 |
| [4] |
MO S H. Research progress of Fig[J]. Prim J Chin Mat Med, 1998, 12(2): 54-56. 无花果研究进展[J]. 基层中药杂志, 1998, 12(2): 54-56. DOI:10.13728/J.1673-6427.1998.02.043 |
| [5] |
FU G M, LI W J, HUANG X Z, et al. Antioxidant and alpha-gluco- sidase inhibitory activities of isoflavonoids from the rhizomes of Ficus tikoua Bur.[J]. Nat Prod Res, 2018, 32(4): 399-405. DOI:10.1080/14786419.2017.1312391 |
| [6] |
TIAN M Y, ZHAO X G, WU X H, et al. Chemical composition, anti- bacterial and cytotoxic activities of the essential oil from Ficus tikoua Bur.[J]. Rec Nat Prod, 2020, 14(3): 219-224. DOI:10.25135/RNP.161.19.10.1450 |
| [7] |
CHENG J X, ZHANG B D, ZHU W F, et al. Traditional uses, phyto- chemistry, and pharmacology of Ficus hispida L. f. : A review[J]. J Ethnopharmacol, 2020, 248: 112204. DOI:10.1016/j.jep.2019.112204 |
| [8] |
BADGUJAR S B, PATEL V V, BANDIVDEKAR A H, et al. Tradi- tional uses, phytochemistry and pharmacology of Ficus carica: A review[J]. Pharm Biol, 2014, 52(11): 1487-1503. DOI:10.3109/13880209.2014.892515 |
| [9] |
WANG Y X, ZHANG X L, GAO L, et al. Antitumor activity of Ficus[J]. Cancer, 1990, 9(3): 223-225. 无花果抗癌作用的研究[J]. 癌症, 1990, 9(3): 223-225. |
| [10] |
VAYA J, MAHMOOD S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.)[J]. Biofactors, 2006, 28(3/4): 169-175. |
| [11] |
LI Z Y, YANG Y, LIU M M, et al. A comprehensive review on phyto- chemistry, bioactivities, toxicity studies, and clinical studies on Ficus carica Linn. leaves[J]. Biomed Pharmacoth, 2021, 137: 111393. DOI:10.1016/j.biopha.2021.111393 |
| [12] |
XU X K, HU J, LIU R H, et al. Study of the chemical constituents of radix of Ficus caric L.[J]. Pharm Care Res, 2005, 5(2): 138-140. 无花果根化学成分研究[J]. 药学服务与研究, 2005, 5(2): 138-140. |
| [13] |
ALI M, CHAUDHARY N. Ficus hispida Linn. : A review of its pharmacognostic and ethnomedicinal properties[J]. Pharmacognosy Rev, 2011, 5(9): 96. DOI:10.4103/0973-7847.79104 |
| [14] |
AWOUAFACK M D, WONG C P, TANE P, et al. Prenylated flavor- noids in food[J]. Handbook Diet Phytochem, 2020, 1-23. DOI:10.1007/978-981-13-1745-3_12-1 |
| [15] |
YANG X M, JIANG Y M, YANG J L, et al. Prenylated flavonoids, promising nutraceuticals with impressive biological activities[J]. Trends Food Sci Technol, 2015, 44(1): 93-104. DOI:10.1016/j.tifs.2015.03.007 |
| [16] |
YANG J L, WEN L R, JIANG Y M, et al. Natural estrogen receptor modulators and their heterologous biosynthesis[J]. Trends Endocrinol, 2019, 30(1): 66-76. DOI:10.1016/j.tem.2018.11.002 |
| [17] |
MONTEIRO N E S, QUEIRÓS L D, LOPES D B, et al. Impact of microbiota on the use and effects of isoflavones in the relief of climac- teric symptoms in menopausal women: A review[J]. J Funct Foods, 2018, 41: 100-111. DOI:10.1016/j.jff.2017.12.043 |
| [18] |
PARHIZ H, ROOHBAKHSH A, SOLTANI F, et al. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models[J]. Phytother Res, 2015, 29(3): 323-331. DOI:10.1002/ptr.5256 |
| [19] |
MIGUEL M G. Antioxidant activity of medicinal and aromatic plants: A review[J]. Flavour Fragr J, 2010, 25(5): 291-312. DOI:10.1002/ffj.1961 |
| [20] |
SAKAT S S, JUVEKAR A R. Comparative study of Erythrina indica Lam. (Febaceae) leaves extracts for antioxidant activity[J]. J Young Pharm, 2010, 2(1): 63-67. DOI:10.4103/0975-1483.62216 |
| [21] |
NAMKOONG S, KIM T J, JANG I S, et al. Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-κB pathways in lung tumor cells[J]. Biol Pharm Bull, 2011, 34(2): 203-208. DOI:10.1248/bpb.34.203 |
| [22] |
PROCHÁZKOVÁ D, BOUŠOVÁ I, WILHELMOVÁ N. Antioxidant and prooxidant properties of flavonoids[J]. Fitoterapia, 2011, 82(4): 513-523. DOI:10.1016/j.fitote.2011.01.018 |
| [23] |
POPOOLA O K, MARNEWICK J L, RAUTENBACH F, et al. Inhibition of oxidative stress and skin aging-related enzymes by preny- lated chalcones and other flavonoids from Helichrysum teretifolium[J]. Molecules, 2015, 20(4): 7143-7155. DOI:10.3390/molecules20047143 |
| [24] |
MALEKI S J, CRESPO J F, CABANILLAS B. Anti-inflammatory effects of flavonoids[J]. Food Chem, 2019, 299: 125124. DOI:10.1016/j.foodchem.2019.125124 |
| [25] |
LIU Y P, GUO J M, YAN G, et al. Anti-inflammatory and antipro- liferative prenylated isoflavone derivatives from the fruits of Ficus carica[J]. J Agric Food Chem, 2019, 67(17): 4817-4823. DOI:10.1021/acs.jafc.9b00865 |
| [26] |
LEE J, KIM B Y, SON Y, et al. 4ʹ-O-Methylalpinumisoflavone inhibits the activation of monocytes/macrophages to an immunostimulatory phenotype induced by 27-hydroxycholesterol[J]. Int J Mol Med, 2019, 43(5): 2177-2186. DOI:10.3892/IJMM.2019.4135 |
| [27] |
WANG Z G, HE D, JIN H, et al. The research progress of the anti- cancer effect of fig[J]. Prog Mod Biomed, 2010, 10(11): 2183-2186. 无花果抗癌作用的研究进展[J]. 现代生物医学进展, 2010, 10(11): 2183-2186. DOI:10.13241/j.cnki.pmb.2010.11.015 |
| [28] |
WEN L R, ZHOU T, JIANG Y M, et al. Prenylated flavonoids in foods and their applications on cancer prevention[J]. Crit Rev Food Sci Nutri, 2021, 1-14. DOI:10.1080/10408398.2021.1881437 |
| [29] |
KANG M J, KIM S Y, KWON E B, et al. Derrone induces autophagic cell death through induction of ROS and ERK in A549 cells[J]. PLoS One, 2019, 14(6): e0218659. DOI:10.1371/journal.pone.0218659 |
| [30] |
ZHANG H R, WU X W, WANG J X, et al. Flavonoids from the leaves of Epimedium koreanum Nakai and their potential cytotoxic activities[J]. Nat Prod Res, 2020, 34(9): 1256-1263. DOI:10.1080/14786419.2018.1560283 |
| [31] |
NAYYAR A, JAIN R. Recent advances in new structural classes of anti-tuberculosis agents[J]. Curr Med Chem, 2005, 12(16): 1873-1886. DOI:10.2174/0929867054546654 |
| [32] |
REN J, HUANG Q H, XU Y Y, et al. Isoflavone lupiwighteone induces cytotoxic, apoptotic, and antiangiogenic activities in DU-145 prostate cancer cells[J]. Anti-Cancer Drugs, 2015, 26(6): 599-611. DOI:10.1097/CAD.0000000000000224 |
| [33] |
DZOYEM J P, HAMAMOTO H, NGAMENI B, et al. Antimicrobial action mechanism of flavonoids from Dorstenia species[J]. Drug Discov Ther, 2013, 7(2): 66-72. DOI:10.5582/ddt.2013.v7.2.66 |
| [34] |
LOPES G, PINTO E, SALGUEIRO L. Natural products: An alternative to conventional therapy for dermatophytosis?[J]. Mycopathologia, 2017, 182(1/2): 143-167. DOI:10.1007/s11046-016-0081-9 |
| [35] |
DAI J Q, SHEN D, YOSHIDA W Y, et al. Isoflavonoids from Ficus benjamina and their inhibitory activity on BACE1[J]. Planta Med, 2012, 78(12): 1357-1362. |
| [36] |
NKENGFACK A E, WAFFO A K, AZEBAZE G A, et al. Indicanine A, a new 3-phenylcoumarin from root bark of Erythrina indica[J]. J Nat Prod, 2000, 63(6): 855-856. DOI:10.1021/np990300y |
| [37] |
MAGALHÃES A F, TOZZI A M G A, MAGALHÃES E G, et al. New prenylated metabolites of Deguelia longeracemosa and evaluation of their antimicrobial potential[J]. Planta Med, 2006, 72(4): 358-363. DOI:10.1055/s-2005-916232 |
| [38] |
ULLAH S, HUSSAIN S, KHAN S N, et al. The medicinal plants in the control of tuberculosis: Laboratory study on medicinal plants from the northern area of Pakistan[J]. Int J Mycobacteriol, 2017, 6(1): 102. DOI:10.4103/ijmy.ijmy_11_17 |
| [39] |
HAN Y T, YANG X W, ZHAO N, et al. Alpinumisoflavone induces apoptosis in esophageal squamous cell carcinoma by modulating miR- 370/PIM1 signaling[J]. Am J Cancer Res, 2016, 6(12): 2755-2771. |
| [40] |
JIANG X W, CAO C T, SUN W W, et al. Scandenolone from Cudrania tricuspidata fruit extract suppresses the viability of breast cancer cells (MCF-7) in vitro and in vivo[J]. Food Chem Toxicol, 2019, 126: 56-66. DOI:10.1016/j.fct.2019.02.020 |
| [41] |
LANSKY E P, PAAVILAINEN H M, PAWLUS A D, et al. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents[J]. J Ethnopharmacol, 2008, 119(2): 195-213. DOI:10.1016/j.jep.2008.06.025 |
| [42] |
MATSUDA H, MORIKAWA T, XU F M, et al. New isoflavones and pterocarpane with hepatoprotective activity from the stems of Erycibe expansa[J]. Planta Med, 2004, 70(12): 1201-1209. DOI:10.1055/s-2004-835852 |
| [43] |
SHIN J H, IRFAN M, RHEE M H, et al. Antiplatelet effect of cudra- xanthone B is related to inhibition of calcium mobilization, αIIbβ3 activation, and clot retraction[J]. Appl Biol Chem, 2021, 64(1): 4. DOI:10.1186/s13765-020-00575-1 |
| [44] |
MERIANE D, GENTA-JOUVE G, KAABECHE M, et al. Rapid identi- fication of antioxidant compounds of Genista saharae Coss. & Dur. by combination of DPPH scavenging assay and HPTLC-MS[J]. Mole- cules, 2014, 19(4): 4369-4379. DOI:10.3390/molecules19044369 |
| [45] |
KALLI S, ARAYA-CLOUTIER C, DE BRUIJN W J C, et al. Induction of promising antibacterial prenylated isoflavonoids from different subclasses by sequential elicitation of soybean[J]. Phytochemistry, 2020, 179: 112496. DOI:10.1016/j.phytochem.2020.112496 |
| [46] |
SHI Z F, LEI C, YU B W, et al. New alkaloids and α-glucosidase inhibitory flavonoids from Ficus hispida[J]. Chem Biodiv, 2016, 13(4): 445-450. DOI:10.1002/CBDV.201500142 |
| [47] |
KANG L, ZHOU J X, SHEN Z W. Two novel antibacterial flavonoids from Myrsine africana L.[J]. Chin J Chem, 2007, 25(9): 1323-1325. DOI:10.1002/CJOC.200790245 |
| [48] |
BADADHE P V, PATIL L R, BHAGAT S S, et al. Synthesis and antimicrobial screening of some novel chromones and pyrazoles with incorporated isoxazole moieties[J]. J Heterocyclic Chem, 2013, 50(5): 999-1004. DOI:10.1002/JHET.1545 |
| [49] |
TISTAERT C, DEJAEGHER B, CHATAIGNÉ G, et al. Potential antioxidant compounds in Mallotus species fingerprints, Part II: fingerprint alignment, data analysis and peak identification[J]. Anal Chim Acta, 2012, 721: 35-43. DOI:10.1016/j.aca.2012.01.058 |
| [50] |
SASAKI H, KASHIWADA Y, SHIBATAV H, et al. Prenylated flavonoids from the roots of Desmodium caudatum and evaluation of their antifungal activity[J]. Planta Med, 2012, 78(17): 1851-1856. DOI:10.1055/s-0032-1315391 |
| [51] |
MOLLAZADEH S, NESHATI V, BAZZAZ B S F, et al. Standardized Sophora pachycarpa root extract enhances osteogenic differentiation in adipose-derived human mesenchymal stem cells[J]. Phytother Res, 2017, 31(5): 792-800. DOI:10.1002/PTR.5803 |
| [52] |
OMISORE N O A, ADEWUNMI C O, IWALEWA E O, et al. Antitri- chomonal and antioxidant activities of Dorstenia barteri and Dorstenia convexa[J]. Braz J Med Biol Res, 2005, 38(7): 1087-1094. DOI:10.1590/S0100-879X2005000700012 |
| [53] |
GÓRNIAK I, BARTOSZEWSKI R, KRÓLICZEWSKI J. Compre- hensive review of antimicrobial activities of plant flavonoids[J]. Phytochem Rev, 2019, 18(1): 241-272. DOI:10.1007/s111BARNES01-018-9591-z |
| [54] |
AKTER K, BARNES E C, LOA-KUM-CHEUNG W L, et al. Anti- microbial and antioxidant activity and chemical characterisation of Erythrina stricta Roxb. (Fabaceae)[J]. J Ethnopharmacol, 2016, 185: 171-181. DOI:10.1016/j.jep.2016.03.011 |
 2022, Vol. 30
2022, Vol. 30



