2. 茅台学院资源环境系, 贵州 仁怀 564507;
3. 贵州茅台酒股份有限公司, 贵州 仁怀 564507;
4. 中国科学院华南植物园, 广州 510650
2. Department of Resources and Environment, Moutai Institute, Renhuai 564507, Guizhou, China;
3. Kweichow Moutai Co. Ltd, Renhuai 564507, Guizhou, China;
4. South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China
交趾黄檀(Dalbergia cochinchinensis)主要产于泰国、老挝、越南、柬埔寨等东南亚地区,其木材表面具有光泽,木质结构均匀且细腻,硬度大, 强度高,具有很强的抗虫性能和耐腐蚀性能,适于制作精美工艺品、高档红木家具、高级乐器等[1-2]。交趾黄檀心材在泰国有作为民间药材使用[3],据报道,其主要含有二氢黄酮、异黄酮、新黄酮、二氢异黄酮、查尔酮、苯并呋喃、酚类等成分。有学者已从交趾黄檀中分离鉴定出65个化合物,包括9个二氢黄酮、8个异黄酮、7个新黄酮、3个二氢异黄酮、5个查尔酮、3个黄烷、2个黄酮、1个异黄烷、6个酚类、5个苯甲酸及苯甲酮类、4个苯并呋喃类、2个甾体类、3个萜类、1个紫檀烷类、2个糖苷及其苷元、1个菲醌类、1个氧杂蒽酮、1个二苯乙烯类、1个叔醇类[4-14]。本课题组在前期研究的基础上进一步对其心材进行化学成分研究,从中分离得到12个化合物。
1 材料和方法 1.1 材料试验材料于2011年由老挝进口,为佛山市家禾木家具有限公司彭庆明提供,由中国科学院华南植物园邓云飞研究员鉴定为交趾黄檀(Dalbergia cochinchinensis)心材。
1.2 仪器和试剂美国Applied Biosystems公司生产的API2000 LC/MS/MS质谱仪;瑞士Bruker公司生产的DRX- 400(500)型核磁共振波谱仪,内标为四甲基硅烷试剂,化学位移δ用ppm表示,偶合常数J用Hz表示。
柱色谱硅胶(100~200和200~300目);柱色谱反相硅胶Rp-C18 (50和70 μm);柱色谱用凝胶Sepha- dex LH-20 (日本三菱);薄层硅胶层析板(烟台黄务);反相硅胶层析板(德国Merck);氯仿、乙酸乙酯、甲醇、盐酸等试剂均为分析纯。
1.3 提取和分离交趾黄檀心材5.0 kg用工业酒精浸提3次,依次为72、48和48 h,减压浓缩后合并得到总浸膏,再溶于适量水中得悬浊液,倒入分液漏斗加入乙酸乙酯溶剂进行萃取,合并3次萃取液再减压浓缩, 最后得到乙酸乙酯部分250.0 g。
取乙酸乙酯部分235.0 g,采用硅胶(100~200目)柱色谱进行分离,依次以正己烷-乙酸乙酯(7∶1~1∶1)为洗脱剂进行梯度洗脱,每次1 L收集洗脱液,各洗脱液减压浓缩后用TLC板检查,合并主点相似洗脱液,共计得到13个组分Fr-1~Fr-13。Fr-2采用硅胶柱色谱分离,以正己烷-乙酸乙酯(50∶1~10∶1)为洗脱剂进行梯度洗脱,收集洗脱液,合并相似组分,共计得到8个亚组分Fr-2-1~Fr-2-8。Fr-2-8采用ODS-C18反相柱层析分离,以97%甲醇-水为流动相,从洗脱液中析出白色针状结晶,即为化合物10 (2.5 mg) (图 1)。Fr-3采用硅胶柱色谱分离, 以正己烷-乙酸乙酯(50∶1~1∶1)为洗脱剂进行梯度洗脱,共计得到6个亚组分Fr-3-1~Fr-3-6。其中Fr-3-4采用凝胶(甲醇)柱色谱等度洗脱得到化合物3 (7.5 mg)。Fr-3-6先后采用凝胶柱色谱(氯仿-甲醇)等度洗脱、ODS反相柱色谱(甲醇-水)梯度洗脱, 得化合物4 (10.2 mg)。Fr-4采用凝胶柱(氯仿-甲醇1∶3)色谱分离,得到7个亚组分Fr-4-1~Fr-4-7。Fr-4-3采用正相硅胶柱色谱、Sephadex LH-20凝胶柱色谱和制备薄层硅胶层析,得到化合物5 (15.3 mg)、6 (18.2 mg)和12 (2.1 mg)。Fr-5采用硅胶柱(氯仿-甲醇100∶1~10∶1)色谱分离,依次梯度洗脱后经过合并得亚组分Fr-5-1~Fr-5-17。有无色结晶从Fr- 5-4中析出来,即为化合物7 (20.0 mg)。Fr-5-6采用硅胶柱色谱分离,经正己烷-乙酸乙酯(4∶1~1∶1)梯度洗脱得13个亚组分,Fr-5-6-10经反复凝胶柱色谱和硅胶柱色谱分离,得到化合物8 (25.5 mg)。Fr-5-6-12采用硅胶柱色谱分离,以正己烷-丙酮(8∶1~3∶1)梯度洗脱得到56个组分,其中Fr-5-6-12-6经硅胶柱色谱分离,以正己烷-乙酸乙酯(4∶1)等度洗脱,得到化合物1 (11.1 mg),Fr-5-6-12-11经凝胶柱(甲醇)层析及薄层硅胶层析板制备得到化合物2 (2.5 mg)和9 (3.2 mg)。Fr-7采用硅胶柱色谱(氯仿-甲醇500∶1~50∶1)和凝胶(甲醇)柱色谱分离,有淡黄色结晶从组分Fr-7-11-18中析出,即为化合物11 (3.8 mg)。
|
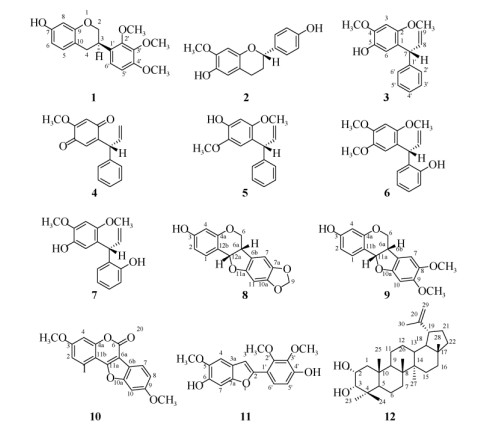
图 1 化合物1~12的结构 Fig. 1 Structures of compounds 1-12 |
化合物1 无色结晶;ESI-MS m/z: 339 [M + Na]+, 655 [2M + Na]+, 315 [M-H]–,分子式为C18H20O5; 1H NMR (400 MHz, CDCl3): δ 6.94 (1H, d, J = 8.1 Hz, H-5), 6.80 (1H, d, J = 8.6 Hz, H-6ʹ), 6.66 (1H, d, J = 8.7 Hz, H-5ʹ), 6.40 (1H, dd, J = 8.1, 2.5 Hz, H-6), 6.37 (1H, d, J = 2.4 Hz, H-8), 5.17 (br s, -OH), 4.28 (1H, ddd, J = 10.4, 3.4, 1.6 Hz, H-2a), 3.99 (1H, t, J = 10.3 Hz, H-2b), 3.90 (3H, s, H-2ʹ), 3.89 (3H, s, H-3ʹ), 3.85 (3H, s, H-4ʹ), 3.54 (1H, m, H-3), 2.89 (2H, m, H-4); 13C NMR (100 MHz, CDCl3): δ 155.1 (C-7), 155.0 (C-9), 152.6 (C-4ʹ), 151.9 (C-2ʹ), 142.3 (C-3ʹ), 130.4 (C-5), 127.3 (C-1ʹ), 121.4 (C-6ʹ), 114.6 (C-10), 108.0 (C-6), 107.5 (C-5ʹ), 103.2 (C-8), 70.4 (C-2), 61.3 (2ʹ-OCH3), 60.8 (3ʹ-OCH3), 56.0 (4ʹ-OCH3), 31.8 (C-3), 31.3 (C-4)。以上数据与文献[15]报道一致, 故鉴定为7-hydroxy-2ʹ, 3ʹ, 4ʹ-trimethoxyisoflavan。
化合物2 无色结晶;ESI-MS m/z: 273 [M + H]+, 295 [M + Na]+, 567 [2M + Na]+, 271 [M-H]–, 分子式为C16H16O4; 1H NMR [500 MHz, (CD3)2CO]: δ 8.58 (1H, s, 4′-OH), 7.27 (2H, d, J = 8.4 Hz, H-2′, 6′), 7.09 (1H, s, 6-OH), 6.84 (2H, d, J = 8.5 Hz, H-3′, 5′), 6.54 (1H, s, H-5), 6.40 (1H, s, H-8), 4.87 (1H, dd, J = 10.3, 2.0 Hz, H-2), 3.78 (3H, s, 7-OCH3), 2.86 (1H, m, H-4a), 2.63 (1H, ddd, J = 16.2, 5.3, 2.9 Hz, H-4b), 2.10 (1H, m, H-3a), 1.96 (1H, m, H-3b); 13C NMR [125 MHz, (CD3)2CO]: δ 158.0 (C-4′), 149.2 (C-7), 147.6 (C-9), 141.2 (C-6), 134.2 (C-1′), 128.3 (C-2′, 6′), 116.0 (C-3′, 5′), 115.9 (C-5), 114.2 (C-10), 101.8 (C- 8), 78.2 (C-2), 56.4 (7-OCH3), 31.0 (C-3), 25.4 (C-4)。以上数据与文献[6]报道基本一致,故鉴定为6, 4ʹ- dihydroxy-7-methoxyflavan。
化合物3 棕色油状固体;ESI-MS m/z: 269 [M-H]–,271 [M + H]+, 293 [M + Na]+, 分子式为C17H18O3; 1H NMR (400 MHz, CDCl3): δ 7.22~7.09 (5H, m, B-ring), 6.65 (1H, s, H-6), 6.43 (1H, s, H-3), 6.18 (1H, ddd, J = 17.0, 10.1, 6.7 Hz, H-8), 5.11 (2H, overlapped, H-9a, -OH), 5.00 (1H, d, J = 6.6 Hz, H-7), 4.84 (1H, d, J = 17.1 Hz, H-9b), 3.81 (3H, s, -OCH3), 3.64 (3H, s, -OCH3); 13C NMR (100 MHz, CDCl3): δ 150.3 (C-2), 144.8 (C-4), 143.0 (C-1′), 140.2 (C-8), 139.1 (C-5), 128.3 (C-3′, 5′), 127.9 (C-2′, 6′), 125.7 (C-4′), 124.4 (C-1), 115.7 (C-9), 115.0 (C-6), 97.1 (C- 3), 56.7 (4-OCH3), 55.9 (2-OCH3), 46.7 (C-7)。以上数据与文献[16]报道一致,故鉴定为R-dalbergiphenol。
化合物4 棕黄色粉末;ESI-MS m/z: 255 [M + H]+, 277 [M + Na]+, 531 [2M + Na]+, 253 [M-H]–, 分子式为C16H14O3; 1H NMR (400 MHz, CDCl3): δ 7.34~7.16 (5H, m, B-ring), 6.49 (1H, s, H-6), 6.10 (1H, ddd, J = 17.0, 10.2, 6.7 Hz, H-8), 5.92 (1H, s, H-3), 5.28 (1H, d, J = 10.2 Hz, H-9a), 5.00 (1H, d, J = 17.2 Hz, H-9b), 3.81 (1H, s, -OCH3), 4.93 (1H, d, J = 6.6 Hz, H-7); 13C NMR (100 MHz, CDCl3): δ 186.5 (C-2), 182.6 (C-5), 158.7 (C-4), 151.2 (C-1), 139.5 (C-1′), 137.4 (C-8), 131.8 (C-4′), 129.0 (C-3′, 5′), 128.8 (C-2′, 6′), 127.4 (C-6), 118.4 (C-9), 108.1 (C-3), 56.5 (4-OCH3), 47.2 (C-7)。以上数据与文献[17]报道一致,故鉴定为R-4-methoxydalbergione。
化合物5 棕黄色油状固体;ESI-MS m/z: 271 [M + H]+, 293 [M + Na]+, 563 [2M + Na]+, 269 [M-H]–,分子式为C17H18O3; 1H NMR (400 MHz, CDCl3): δ 7.39~7.17 (5H, m, B-ring), 6.58 (1H, s, H-6), 6.45 (1H, s, H-3), 6.31 (1H, ddd, J = 16.9, 10.2, 6.5 Hz, H-8), 5.30 (1H, d, J = 10.2 Hz, H-9a), 5.02 (1H, d, J = 17.2 Hz, H-9b), 4.88 (2H, overlapped, H-7, -OH), 3.80 (3H, s, 2-OCH3), 3.75 (3H, s, 5-OCH3); 13C NMR (100 MHz, CDCl3): δ 148.5 (C-2), 147.6 (C-4), 142.9 (C-5), 141.5 (C-1ʹ), 139.5 (C-8), 128.6 (C-3ʹ, 5ʹ), 128.5 (C-2ʹ, 6ʹ), 126.7 (C-4ʹ), 119.7 (C-1), 116.9 (C-9), 113.3 (C-6), 101.6 (C-3), 56.5 (5-OCH3), 55.8 (2- OCH3), 48.9 (C-7)。以上数据与文献[18]报道一致, 故鉴定为mimosifoliol。
化合物6 无色结晶;ESI-MS m/z: 301 [M + H]+, 323 [M + Na]+, 623 [2M + Na]+, 299 [M-H]–, 分子式为C18H20O4; 1H NMR (400 MHz, CDCl3): δ 7.18~7.10 (2H, m, B-ring), 6.90~6.83 (2H, m, B-ring), 6.69 (1H, s, H-6), 6.55 (1H, s, H-3), 6.34 (1H, m, H-8), 6.06 (1H, s, 2ʹ-OH), 5.29 (1H, d, J = 10.3 Hz, H-9a), 5.22 (1H, d, J = 5.6 Hz, H-7), 5.04 (1H, d, J = 17.2 Hz, H-9b), 3.76 (3H, s, -OCH3), 3.87 (3H, s, -OCH3), 3.86 (3H, s, -OCH3); 13C NMR (100 MHz, CDCl3): δ 150.1 (C-2), 153.8 (C-2ʹ), 148.5 (C-4), 143.7 (C-5), 139.0 (C-8), 129.2 (C-1ʹ), 128.3 (C-4ʹ), 127.7 (C-6ʹ), 121.3 (C-1), 120.6 (C-5ʹ), 116.8 (C-9), 116.3 (C-3ʹ), 113.2 (C-6), 97.9 (C-3), 57.0 (4-OCH3), 56.6 (5-OCH3), 56.1 (2-OCH3), 40.2 (C-7)。以上数据与文献[19]报道一致,故鉴定为R-5-O-methyllatifolin。
化合物7 无色结晶;ESI-MS m/z: 287 [M + H]+, 309 [M + Na]+, 595 [2M + Na]+, 285 [M-H]–, 分子式为C17H18O4; 1H NMR (400 MHz, CDCl3): δ 7.18~7.10 (2H, m, B-ring), 6.89~6.81 (2H, m, B-ring), 6.75 (1H, s, H-6), 6.52 (1H, s, H-3), 6.32 (1H, m, H-8), 6.05 (1H, s, 2ʹ-OH), 5.32~5.22 (2H, overlapped, H-9a, 5-OH), 5.19 (1H, d, J = 5.8 Hz, H-7), 5.04 (1H, dt, J = 17.1, 1.4 Hz, H-9b), 3.87 (3H, s, -OCH3), 3.85 (3H, s, -OCH3); 13C NMR (100 MHz, CDCl3): δ 153.7 (C-2ʹ), 149.4 (C-2), 145.5 (C-4), 140.1 (C-5), 139.0 (C-8), 129.4 (C-1ʹ), 128.4 (C-4ʹ), 127.7 (C-6ʹ), 122.5 (C-1), 120.6 (C-5ʹ), 116.7 (C-9), 116.3 (C-3ʹ), 115.2 (C-6), 97.0 (C-3), 57.1 (4-OCH3), 56.1 (2-OCH3), 40.0 (C- 7)。以上数据与文献[19]报道一致,故鉴定为R-lati- folin。
化合物8 白色粉末;ESI-MS m/z: 283 [M-H]–, 307 [M + Na]+, 285 [M + H]+, 分子式为C16H12O5; 1H NMR (400 MHz, CDCl3): δ 7.36 (1H, d, J = 8.4 Hz, H-1), 6.72 (1H, s, H-7), 6.55 (1H, dd, J = 8.4, 2.4 Hz, H-2), 6.44 (1H, s, H-11), 6.42 (1H, d, J = 2.4 Hz, H-4), 5.92 (1H, d, J = 0.9 Hz, H-9α), 5.90 (1H, d, J = 0.9 Hz, H-9β), 5.47 (1H, d, J = 6.9 Hz, H-12a), 5.42 (1H, s, -OH), 4.22 (1H, dd, J = 11.0, 5.0 Hz, H-6α), 3.64 (1H, t, J = 11.0 Hz, H-6β), 3.47 (1H, m, H-6a); 13C NMR (100 MHz, CDCl3) δ: 157.1 (C-3), 156.6 (C-4a), 154.2 (C-11a), 148.1 (C-10a), 141.7 (C-7a), 132.1 (C-1), 117.9 (C-6b), 112.6 (C-12b), 109.8 (C-2), 104.7 (C-7), 103.6 (C-4), 101.3 (C-9), 93.8 (C-11), 78.5 (C-12a), 66.4 (C-6), 40.1 (C-6a)。以上数据与文献[20]报道基本一致,故鉴定为maackiain。
化合物9 棕色粉末;ESI-MS m/z: 301 [M + H]+, 323 [M + Na]+, 623 [2M + Na]+, 299 [M-H]–, 分子式为C17H16O5; 1H NMR [500 MHz, (CD3)2CO]: δ 8.75 (1H, s, 3-OH), 7.29 (1H, d, J = 8.4 Hz, H-1), 7.00 (1H, s, H-7), 6.55 (1H, dd, J = 8.4, 2.4 Hz, H-2), 6.48 (1H, s, H-10), 6.36 (1H, d, J = 2.3 Hz, H-4), 5.46 (1H, d, J = 7.0 Hz, H-11a), 4.26 (1H, dd, J = 10.6, 4.5 Hz, H-6α), 3.75 (3H, s, 9-OCH3), 3.74 (3H, s, 8-OCH3), 3.56 (1H, m, H-6a), 3.63 (1H, m, H-6β); 13C NMR [125 MHz, (CD3)2CO]: δ 159.8 (C-3), 157.8 (C-4a), 155.1 (C-10a), 151.8 (C-9), 145.0 (C-8), 133.1 (C-1), 118.7 (C-6b), 113.1 (C-11b), 111.6 (C-7), 110.6 (C-2), 104.1 (C-4), 96.9 (C-10), 79.1 (C-11a), 67.2 (C-6), 57.7 (8-OCH3), 56.5 (9-OCH3), 41.4 (C-6a)。以上数据与文献[21]报道一致,故鉴定为secundiflorol Ι。
化合物10 白色针状结晶;ESI-MS m/z: 297 [M + H]+, 319 [M + Na]+, 615 [2M + Na]+, 295 [M-H]–,分子式为C17H12O5; 1H NMR (400 MHz, CDCl3): δ 7.96 (1H, d, J = 8.6 Hz, H-1), 7.89 (1H, d, J = 8.4 Hz, H-7), 7.18 (1H, d, J = 1.8 Hz, H-10), 7.05 (1H, dd, J = 8.4, 2.0 Hz, H-8), 6.99 (2H, m, H-2, 4), 3.92 (3H, s, -OCH3), 3.91 (3H, s, -OCH3); 13C NMR (100 MHz, CDCl3): δ 162.6 (C-3), 160.1 (C-11a), 159.2 (C-9), 158.5 (C-6), 156.5 (C-10a), 155.1 (C-4a), 122.4 (C-7), 121.6 (C-1), 116.6 (C-6b), 113.2 (C-2), 113.0 (C-8), 106.1 (C-11b), 103.5 (C-6a), 101.4 (C-4), 96.8 (C-10), 55.9 (-OCH3), 55.8 (-OCH3)。以上数据与文献[22]报道一致,故鉴定为3, 9-dimethoxy-6H-benzofufo[3, 2-c] chromen-6-one。
化合物11 黄色粉末;ESI-MS m/z: 317 [M + H]+, 339 [M + Na]+, 655 [2M + Na]+, 315 [M-H]–, 分子式为C17H16O6; 1H NMR (400 MHz, DMSO-d6): δ 7.24 (1H, d, J = 8.8 Hz, H-6ʹ), 7.04 (1H, s, H-4), 6.99 (1H, s, H-3), 6.93 (1H, s, H-7), 6.73 (1H, d, J = 8.8 Hz, H-5ʹ), 3.78 (3H, s, 5-OCH3), 3.81 (3H, s, 2ʹ- OCH3), 3.80 (3H, s, 3ʹ-OCH3); 13C NMR (100 MHz, DMSO-d6): δ 148.6 (C-2ʹ), 150.4 (C-4ʹ), 148.2 (C-2), 145.2 (C-7a), 145.0 (C-5), 144.6 (C-6), 139.7 (C-3ʹ), 120.5 (C-3a), 117.2 (C-1ʹ), 115.4 (C-6ʹ), 107.2 (C-5ʹ), 103.8 (C-3), 102.6 (C-4), 97.7 (C-7), 58.9 (3ʹ-OCH3), 56.0 (2ʹ-OCH3), 55.8 (5-OCH3)。以上数据与文献[23]报道一致,故鉴定为mucodianin C。
化合物12 白色粉末;ESI-MS m/z: 441 [M-H]–, 465 [M + Na]+, 907 [2M + Na]+, 分子式为C30H50O2; 1H NMR (400 MHz, CDCl3): δ 4.57 (1H, s, H-29b), 4.69 (1H, d, J = 2.0 Hz, H-29a), 3.98 (1H, ddd, J = 14.7, 7.1, 4.1 Hz, H-2), 3.42 (1H, s, H-3), 2.38 (1H, td, J = 11.0, 5.8 Hz, H-19), 2.04 (1H, d, J = 2.8 Hz, H-21), 1.69 (3H, s, H-30), 1.02 (3H, s, H-26), 1.00 (3H, s, H-27), 0.95 (3H, s, H-24), 0.88 (3H, s, H-25), 0.84 (3H, s, H-23), 0.78 (3H, s, H-28); 13C NMR (100 MHz, CDCl3): δ 150.9 (C-20), 109.4 (C-29), 79.0 (C-3), 66.7 (C-2), 50.1 (C-9), 48.3 (C-5), 48.2 (C-18), 48.0 (C-19), 43.0 (C-14), 42.9 (C-17), 42.1 (C-1), 41.0 (C-8), 40.0 (C-22), 38.6 (C-10), 38.3 (C-4), 38.0 (C-13), 35.6 (C- 16), 34.0 (C-7), 29.8 (C-21), 28.5 (C-23), 27.4 (C-15), 25.0 (C-12), 21.6 (C-24), 20.9 (C-11), 19.3 (C-30), 18.0 (C-6, 28), 17.1 (C-25), 16.0 (C-26), 14.6 (C-27)。以上数据与文献[24-25]报道基本一致,故鉴定为lup-(20) 29-ene-2α, 3α-diol。
2 结果和讨论从交趾黄檀心材中分离鉴定得到12个化合物, 分别为:7-hydroxy-2ʹ, 3ʹ, 4ʹ-trimethoxyisoflavan (1), 6, 4ʹ-dihydroxy-7-methoxyflavan (2), R-dalbergiphenol (3), R-4-methoxydalbergione (4), mimosifoliol (5), R- 5-O-methyllatifolin (6), R-latifolin (7), maackiain (8), secundiflorol Ι (9), 3, 9-dimethoxy-6H-benzofufo [3, 2-c] chromen-6-one (10), mucodianin C (11)和lup-(20) 29-ene-2α, 3α-diol (12)。化合物1、5、8~12为首次从交趾黄檀心材中分离得到。
在抗骨质疏松试验中,化合物3能显著提高骨细胞的矿化能力,显著上调BMP-2和RunX2的mRNA水平,并能显著提高骨钙素和I型胶原的mRNA表达水平[26]。化合物4、6和7能显著抑制5α-还原酶活性,并能竞争性抑制二氢睾酮与受体的形成,从而有望治疗雄激素活性过高所致的疾病, 包括多毛症、痤疮、前列腺肥大、前列腺癌等[5]。化合物4对β-葡萄糖醛酸苷酶和NO生成均表现出不错的抑制效果,提示其具有较强的抗炎活性。此外,有报道指出,化合物4还具有抗肿瘤、抗过敏、抗菌及疟原虫抑制活性[27]。化合物5在DNA链断裂试验中显示出较弱的活性[18]。本课题从交趾黄檀中分离得到的这5个已知新黄酮类成分,特征性明显,生物活性多样,将为进一步发掘该属植物中潜在的新药资源提供参考价值。
| [1] |
LIN G W. The kind of main wood that national standard mahogany included[J]. For Ecol, 2010(3): 24. 林国网. 国标红木包括哪些主要木材[J]. 湖南林业, 2010(3): 24. DOI:10.13552/j.cnki.lyyst.2010.03.016 |
| [2] |
WU P Y, ZHANG Y B, ZHANG J W. A new rosewood species: Dalbergia cochinchinensis[J]. Fujian Sci Technol Trop Crops, 2016, 41(4): 51-54. 吴培衍, 张荣标, 张金文. 红木树种新贵——交趾黄檀[J]. 福建热作科技, 2016, 41(4): 51-54. DOI:10.3969/j.issn.1006-2327.2016.04.016 |
| [3] |
Palasuwan A, Soogarun S, Lertlum T, et al. Inhibition of heinz body induction in an in vitro model and total antioxidant activity of medicinal thai plants[J]. Asian Pac J Cancer Prev, 2005, 6(4): 458-463. |
| [4] |
DONNELLY D M X, NANGLE B J, PRENDERGAST J P, et al. Dalbergia species: V. Isolation of R-5-O-methyllatifolin from Dalbergia cochinchinensis Pierre.[J]. Phytochemistry, 1968, 7(4): 647-649. DOI:10.1016/S0031-9422(00)88241-2 |
| [5] |
KUROYANAGI M, UENO A, HIRAYAMA Y, et al. Anti-androgen active constituents from Dalbergia cochinchinensis Pierre[J]. Nat Med (Tokyo, Jpn), 1996, 50(6): 408-412. |
| [6] |
PATHAK V, SHIROTA O, SEKITA S, et al. Antiandrogenic phenolic constituents from Dalbergia cochinchinensis[J]. Phytochemistry, 1997, 46(7): 1219-1223. DOI:10.1016/S0031-9422(97)80015-5 |
| [7] |
SVASTI J, SRISOMSAP C, TECHASAKUL S, et al. Dalcochinin-8'-O-β-d-glucoside and its β-glucosidase enzyme from Dalbergia cochin-chinensis[J]. Phytochemistry, 1999, 50(5): 739-743. DOI:10.1016/S0031-9422(98)00552-4 |
| [8] |
SHIROTA O, PATHAK V, SEKITA S, et al. Phenolic constituents from Dalbergia cochinchinensis[J]. J Nat Prod, 2003, 66(8): 1128-1131. DOI:10.1021/np0300683 |
| [9] |
AREE T, TIP-PYANG S, SEESUKPHRONRARAK S, et al. 2-(5, 7-Dihydroxy-4-oxo-4H-chromen-3-yl)-5-methoxy-1, 4-benzoquinone iso-flavonequinone)[J]. Acta Crystallogr Sect E: Struct Rep Online, 2003, E59(3): o363-o365. DOI:10.1107/S1600536803003684 |
| [10] |
AREE T, TIP-PYANG S, PARAMAPOJN S, et al. 3, 9-Dimethoxy-6a, 11a-dihydro-6H-benzo[4, 5] furo[3, 2-c]chromene-4, 10-diol monohydrate[J]. Acta Crystallogr Sect E: Struct Rep Online, 2003, E59(3): o381-o383. DOI:10.1107/S1600536803004227 |
| [11] |
LIU R H, WEN X C, LI Y Y, et al. Chemical constituents from Dalbergia cochinchinensis[J]. J. Chin Med Mat, 2015, 38(9): 1868-1871. 刘荣华, 温新潮, 李于益, 等. 交趾黄檀化学成分研究[J]. 中药材, 2015, 38(9): 1868-1871. DOI:10.13863/j.issn1001-4454.2015.09.017 |
| [12] |
LIU R H, WEN X C, ZHANG P Z, et al. Chemical constituents of isofla-vonoids from Dalbergia cochinchinensis[J]. Chin Trad Herb Drugs, 2015, 46(19): 2851-2855. 刘荣华, 温新潮, 张普照, 等. 交趾黄檀异黄酮类化学成分研究[J]. 中草药, 2015, 46(19): 2851-2855. DOI:10.7501/j.issn.0253-2670.2015.19.006 |
| [13] |
LIU R H, WEN X C, SHAO F, et al. Flavonoids from heartwood of Dalbergia cochinchinensis[J]. Chin Herb Med, 2016, 8(1): 89-93. DOI:10.1016/S1674-6384(16)60014-X |
| [14] |
LIU R H, LI Y Y, SHAO F, et al. A new chalcone from the heartwood of Dalbergia cochinchinensis[J]. Chem Nat Compd, 2016, 52(3): 405-408. DOI:10.1007/s10600-016-1659-7 |
| [15] |
EL-SEBAKHY N A, ASAAD A M, ABDALLAH R M, et al. Anti-microbial isoflavans from Astragalus species[J]. Phytochemistry, 1994, 36(6): 1387-1389. DOI:10.1016/S0031-9422(00)89728-9 |
| [16] |
MUANGNOICHAROEN N, FRAHM A W. Neoflavanoids of Dal-bergia parviflora[J]. Phytochemistry, 1982, 21(3): 767-772. DOI:10.1016/0031-9422(82)83184-1 |
| [17] |
KHAN W N, LODHI M A, ALI I, et al. New natural urease inhibitors from Ranunculus repens[J]. J Enzyme Inhib Med Chem, 2006, 21(1): 17-19. DOI:10.1080/14756360500319210 |
| [18] |
FULLAS F, KORNBERG L J, WANI M C, et al. Two new aromatic constituents from the rootwood of Aeschynomene mimosifolia[J]. J Nat Prod, 1996, 59(2): 190-192. DOI:10.1021/np960052v |
| [19] |
SEKINE N, ASHITANI T, MURAYAMA T, et al. Bioactivity of latifolin and its derivatives against termites and fungi[J]. J Agric Food Chem, 2009, 57(13): 5707-5712. DOI:10.1021/jf900719p |
| [20] |
PARK J A, JA KIM H, JIN C, et al. A new pterocarpan, (-)-maackiain sulfate, from the roots of Sophora subprostrata[J]. Arch Pharm Res, 2003, 26(12): 1009-1013. DOI:10.1007/BF02994750 |
| [21] |
TIAN F, MCLAUGHLIN J L. Bioactive flavonoids from the black locust tree, Robinia pseudoacacia[J]. Pharm Biol, 2000, 38(3): 229-234. DOI:10.1076/1388-0209(200007)3831-SFT229 |
| [22] |
TANG L N, PANG Y L, YAN Q, et al. Synthesis of coumestan deriva-tives via FeCl3-mediated oxidative ring closure of 4-gydroxy coumarins[J]. J Org Chem, 2011, 76(8): 2744-2752. DOI:10.1021/jo2000644 |
| [23] |
GONG T, WANG D X, YANG Y, et al. A novel 3-arylcoumarin and three new 2-arylbenzofurans from Mucuna birdwoodiana[J]. Chem Pharm Bull, 2010, 58(2): 254-256. DOI:10.1248/cpb.58.254 |
| [24] |
KUMAR N, SESHADRI T R. Triterpenoids of Pterocarpus santalinus: constitution of a new lupene diol[J]. Phytochemistry, 1975, 14(2): 521-523. DOI:10.1016/0031-9422(75)85121-1 |
| [25] |
PAN Z H, WANG Y Y, LI M M, et al. Terpenoids from Salvia trijuga[J]. J Nat Prod, 2010, 73(6): 1146-1150. DOI:10.1021/np100250w |
| [26] |
KUMAR P, KUSHWAHA P, KHEDGIKAR V, et al. Neoflavonoids as potential osteogenic agents from Dalbergia sissoo heartwood[J]. Bioorg Med Chem Lett, 2014, 24(12): 2664-2668. DOI:10.1016/j.bmcl.2014.04.056 |
| [27] |
LIU R H, LIN S, ZHANG P Z, et al. Neoflavonoids and their phar-macological activities in Dalbergia genus[J]. China J Chin Mat Med, 2017, 42(24): 4707-4715. 刘荣华, 林帅, 张普照, 等. 黄檀属植物新黄酮类化学成分与药理活性研究进展[J]. 中国中药杂志, 2017, 42(24): 4707-4715. DOI:10.19540/j.cnki.cjcmm.20170928.013 |
 2021, Vol. 29
2021, Vol. 29


