b. 江苏大学农业工程学院, 江苏 镇江 212013
b. School of Agricultural Engineering, Jiangsu University, Zhenjiang 212013, Jiangsu, China
丛枝菌根真菌(arbuscular mycorrhizal fungi, AMF)广泛存在于自然界,是土壤生物群落的关键功能组成部分[1],可以和约80%的陆地植物物种根部形成共生体系,并且可以通过不同的机制影响植物的生长发育[2–3],因此,AMF对生态系统的功能有重要贡献[1]。在共生体系中,寄主植物为AMF提供光合作用的产物碳[1],反之,AMF能促进植物养分吸收[4],提高植物环境胁迫耐受力[5-6],抵抗病原体和食草动物的能力等[7]。AMF的菌丝体在植物根部生长并延伸到土壤中,以探索和利用土壤中的营养物质,随后将其输送给寄主植物[8]。有研究也发现菌根共生还可以在营养缺乏条件下促进寄主植物的养分吸收[9]。
对于植物的正常生长发育来说,磷是必不可少的大量元素之一,它参与很多重要的生理生化过程,包括光合作用、糖分解、能量转移、植物内的营养转运等[10]。土壤中的磷元素虽然存储量大,但磷元素易与土壤中的Fe3+、Ca2+、Mg2+和Al3+等阳离子结合为难溶性的磷酸盐,所以其中约95%的磷不能被植物直接吸收利用[11-12]。由此可见,植物对于磷营养的吸收利用率可能决定了其在新环境中能否很好地生存生长。
很多研究表明植物能利用根际微生物来提高对磷的摄取[13-14],而根际微生物中重要的组成部分就是AMF[15]。有研究认为AMF可以通过以下途径提高植物对磷的摄取:(1) AMF与植物共生以及形成的菌根网络扩大养分吸收范围,改善植物个体营养[16];(2) AMF定殖可以刺激寄主植物根部分泌有机酸,如草酸盐或柠檬酸[17];(3) AMF也可能促进P-溶解细菌分泌相关反应酶,例如磷酸酶[18-19]。通过上述过程将土壤有机磷水解成无机磷酸盐(Pi), 利用细胞膜中磷转运蛋白将Pi转移到AMF的菌丝中,进一步通过磷转运蛋白将可生物利用的无机磷酸盐转移到植物根细胞中[20-22]。入侵植物大多都是菌根植物,在新生存环境需要与AMF形成互利共生关系[23],特别是入侵植物在贫瘠环境下生长时, 因此,研究入侵植物与AMF共生关系及对入侵过程产生的作用已经成为入侵植物土壤微生物机制重要的一个研究方面[24]。
原产于南美洲热带地区的南美蟛蜞菊(Wedelia trilobata)被列为世界上100种恶性入侵种之一[25], 是多年生常绿植物[26]。南美蟛蜞菊是我国华南地区常见的入侵植物,生长、蔓延迅速,从而导致华南地区生态受到严重危害[27]。而我国华南地区土壤有效磷含量很低[28],可见南美蟛蜞菊在磷受限的环境中依然能够较好地生长。通过提高资源有效利用率可能是其生长迅速的关键因素之一。有研究表明菊科(Asteraceae)植物大多都是典型的菌根植物[29-31], 在野外南美蟛蜞菊AMF侵染率可达到65%[32],植物与AMF共生形成菌根网络可能有利于植物获取营养[33]。但是目前丛枝菌根真菌对于南美蟛蜞菊在磷营养摄取方面的作用研究依然不足。
本研究以南美蟛蜞菊为宿主植物,设置可溶和难溶无机磷酸盐2种磷源,以无磷做空白对照,分别接种摩西管柄囊霉(Funneliformis mosseae)和地表球囊霉(Glomus versiforme),探讨这2种AMF能否与南美蟛蜞菊形成良好的共生体系,及其对南美蟛蜞菊生长和难溶磷吸收是否有促进作用,为更全面地理解AMF和入侵植物共生体系对植物营养摄取和入侵过程提供依据。
1 材料和方法 1.1 材料南美蟛蜞菊(Wedelia trilobata)茎段采自福建省厦门市,并在江苏大学的温室中扩繁。试验选取生长健康、长势(长短、粗细)一致的茎段,每个茎段保留2个茎节,且均用次氯酸钠溶液(5%)表面消毒灭菌10 min,然后用无菌水洗涤5次备用。
有研究表明,入侵植物根周围分布的AMF以球囊霉属(Glomus)为优势属[16]。本试验采用地表球囊霉(Glomus versiforme, GV)和摩西管柄囊霉(Funneliformis mosseae, FM)作为供试菌剂,菌剂均购自北京农林科学院营养与资源研究所。试验所用GV、FM菌剂为自行扩繁[34]的含培养基质(粒径小于2 mm洗净后的河沙与沸石,1:1混合均匀)、孢子、菌丝和高粱(Sorghum bicolor)侵染根段的混合物,1 g GV菌剂中约含23个孢子;1 g FM菌剂中约含有50个孢子。
本试验设置2种磷源,分别为可溶性的磷酸二氢钾(KH2PO4)和难溶性的羟基磷灰石[Ca5(PO4)3(OH)]。试验试剂均为分析纯,购于国药集团化学试剂有限公司。
1.2 方法本试验采用沙培盆栽种植方式,洗净灭菌烘干的河沙(直径 < 2 mm,无可利用营养)作为培养基质,并放入圆形塑料盆(口径10 cm,底径6 cm,高度9 cm)中。试验设置3个AMF水平:(1)不添加AMF菌剂,盆中装500 g河沙作为基质;(2)基质中添加8.7 g GV菌剂,与491.3 g沙子混合均匀后装入花盆;(3)基质中添加4 g FM菌剂,与496 g沙子混合均匀后装入花盆。其中2个添加AMF的处理, 基质中加入的菌剂不同质量,是为了确保每盆约含200个孢子。每个AMF处理又设置3个磷水平:(1)不添加磷源(Non-P); (2)添加可溶性磷源(KH2PO4, K-P); (3)添加难溶性磷源[Ca5(PO4)3(OH), Ca-P]。磷含量均为30 mg/kg,磷源试剂均以粉末的形式加入,与河沙混合均匀后装入花盆中。因此,本试验共9个处理,每处理设5个重复,共45盆植物。
种植过程:将准备好的植物茎段垂直扦插在花盆的中心位置,每盆插入1个茎段,保持1个茎节埋在沙子中,所有植株均放在相对湿度为70%, 25℃, 光周期为16 h/8 h的温室进行培养。每2 d用适量蒸馏水浇灌植株,为了补充植物生长所需的其他营养元素,每5 d用50 mL改良无磷元素的Hoagland营养液[34]浇灌植株。生长2个月后,收获植株和培养基质,统计菌根侵染率,测量植物各项生长指标和磷含量。
1.3 菌根侵染率的统计依据Phillips[35]和Trouvelot[36]的方法,从根部取出合适的幼根,洗干净,剪成2 cm小段,依次用10% KOH消化, 30% H2O2漂洗, 1% HCl酸化, 0.05%台盼蓝染色,50%乳酸脱色。然后立即在显微镜下观察丛枝、泡囊和菌丝等形态结构,染色后通过显微镜检查以评估AMF定殖的根百分比,侵染率(%)=被侵染的根段数/镜检的总根段数×100%。
1.4 磷含量的测定将植物叶片用去离子水冲洗干净后在65℃下烘干至恒定质量,然后用快速组织细胞破碎仪(天津欧诺仪器股份有限公司)将样品粉碎磨成粉末状后, 进行总磷元素的测量,先称取0.05 g植物叶片样品, 加入浓HNO3和30%的H2O2,然后在密闭式容器中高温高压完成消解,最后利用电感耦合等离子体发射光谱仪(ICPE-9820, 岛津公司)测量总磷元素含量。
1.5 数据的统计分析数据处理使用SPSS 17.0软件,组间整体差异采用双因素方差分析(Two-Way ANOVA),同时采用ANOVA单因素方差分析和及多重比较(Duncan’s test, P < 0.05)不同(AMF、磷)处理下植物生长的差异显著性,制图均使用Origin 2018软件绘制。
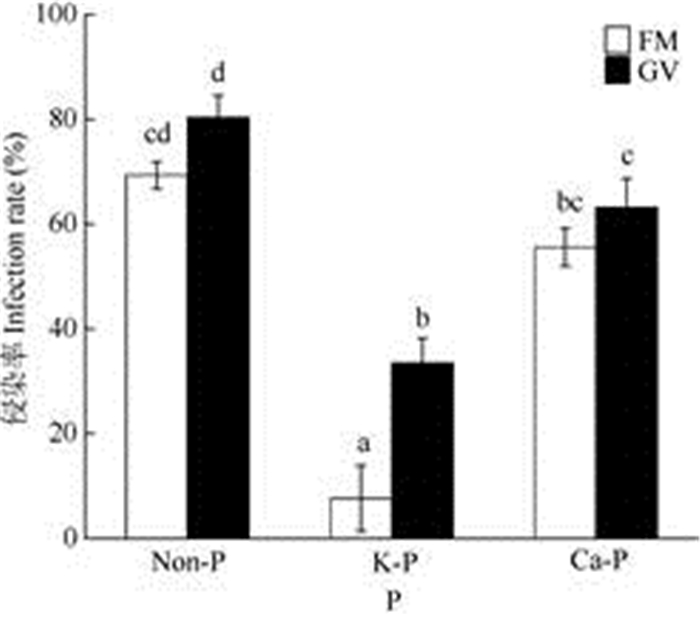
2 结果和分析 2.1 AMF侵染情况不同磷条件下,AMF对南美蟛蜞菊的根部侵染率依次为Non-P (FM: 69.3%; GV: 80.3%) > Ca-P (FM: 55.58%; GV: 63.2%) > K-P (FM: 7.6%; GV: 33%) (图 1);同时,接种GV的根部侵染率均高于接种FM的(图 1)。而未接种AMF的对照并没有发现菌根侵染。
|
图 1 AMF对南美蟛蜞菊根部的侵染率。Non-P:无P; K-P: +KH2PO4; Ca-P: +Ca5(PO4)3(OH); GV: Glomus versiforme; FM: Funneliformis mosseae。下图同。n=5, 柱上不同字母表示差异显著(P < 0.05)。 Fig. 1 Infection rate of Wedelia trilobata roots by AMF. Non-P: No P; K-P: +KH2PO4; Ca-P: +Ca5(PO4)3(OH); GV: Glomus versiforme; FM: Funneli- formis mosseae. n=5. Different letters upon bars indicate significant difference at 0.05 level. |
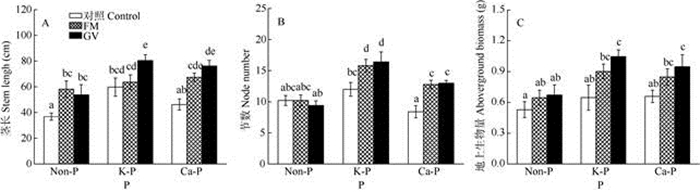
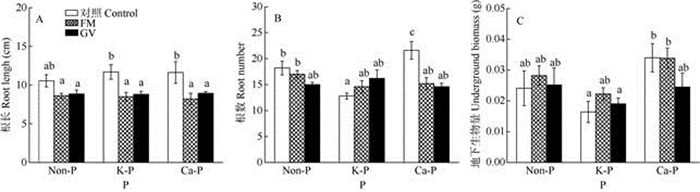
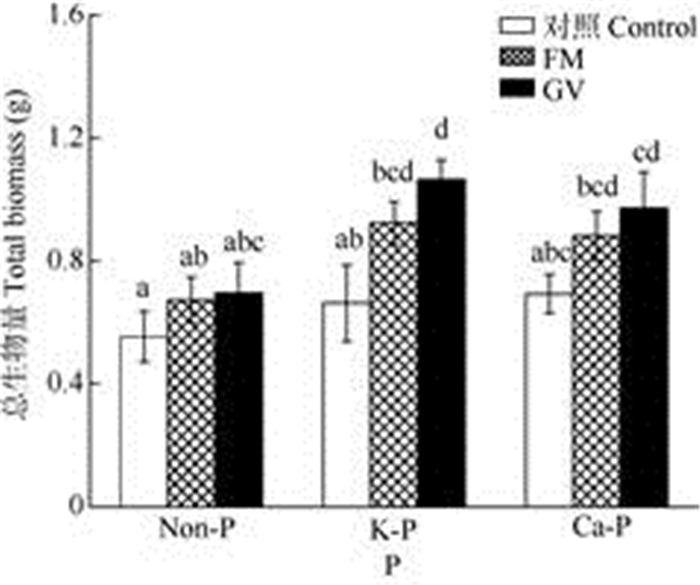
未接种AMF时,K-P处理的南美蟛蜞菊茎长显著高于Non-P和Ca-P处理,单接种AMF的茎长显著增加,对节数、地上生物量的影响并不显著(图 2, 表 1)。在K-P和Ca-P处理下接种GV的植株, 茎长、节数和地上生物量都显著高于未接种AMF的, 接种FM的植株仅节数显著增加,茎长和地上生物量略有增加。对于地下部分,Non-P和Ca-P处理接种AMF后根长显著缩短(图 3: A); 未接种AMF时, Ca-P处理的根数和地上生物量都显著高于K-P处理(图 3: B, C); 接种AMF后根长和根数都显著地降低(图 3: A, B, 表 1),但对地下生物量影响并不显著(图 3: C)。对于总生物量,Ca-P和K-P处理接种AMF后总生物量增加, 其中接种GV的增加40%~60%, Non-P处理接种AMF对植物总生物量并无显著影响(图 4, 表 1)。
| 表 1 磷源和接种AMF对南美蟛蜞菊生长的方差分析 Table 1 ANOVA analysis of phosphorus (P) and AMF on the growth of Wedelia trilobata |
|
图 2 磷和AMF对南美蟛蜞菊茎长(A)、节数(B)和地上生物量(C)的影响 Fig. 2 Effects of P and AMF on stem length (A), node number (B) and aboveground biomass (C) of Wedelia trilobata |
|
图 3 磷和AMF对南美蟛蜞菊根长(A)、根数(B)和地下生物量(C)的影响 Fig. 3 Effects of P and AMF on root length (A), root number (B) and underground biomass (C) of Wedelia trilobata |
|
图 4 磷和AMF对南美蟛蜞菊总生物量的影响 Fig. 4 Effects of P and AMF on total biomass of Wedelia trilobata |
未接种AMF时,K-P处理的叶片磷含量显著高于无磷和Ca-P处理。Ca-P处理时,接种2种AMF的叶片磷含量都显著增加(FM: +36.6%; GV: +40.7%),在Non-P和K-P处理中,接种AMF对叶片磷含量并没有显著影响(图 5)。
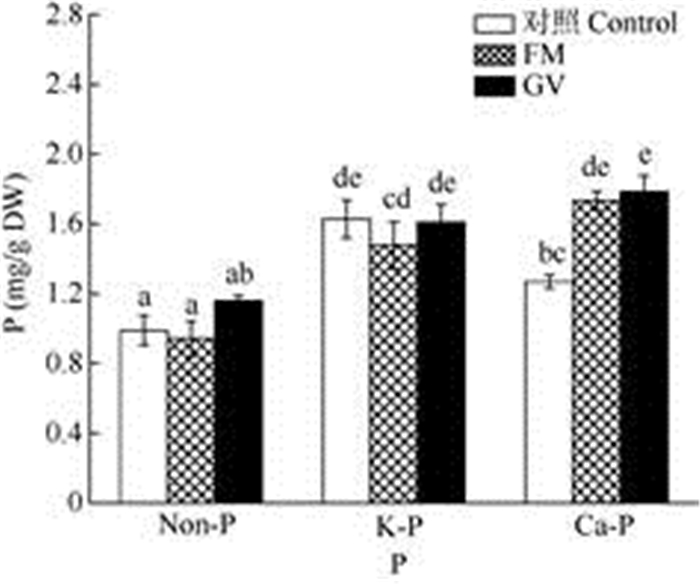
|
图 5 AMF和磷对南美蟛蜞菊叶片磷含量的影响 Fig. 5 Effect of P and AMF on P content in Wedelia trilobata leaves |
本研究结果表明,丛枝菌根真菌FM和GV都能与南美蟛蜞菊在无磷和难溶性磷环境下形成良好的共生关系,FM和GV的侵染率在无磷和难溶性磷处理下显著高于可溶性磷处理,这与Watts- Williams等[37]和Balzergue等[38]报道的在缺磷营养环境AMF侵染率会提高的研究结果一致。且GV的侵染率高于FM。GV和FM侵染率不同可能是因为不同AMF物种的特性和植物物种对某种AMF具有种类偏好性,导致不同的定殖密度[33]。土壤肥力被认为是影响菌根结合的重要因素,低营养环境限制植物生长发育,会增加植物对菌根依赖,植物可以利用菌根网络来吸收更多营养物质[39]。磷缺乏可以诱导植物与AMF共生[40],在我们的研究中,AMF侵染情况受土壤磷营养有效性的影响,如土壤磷有效性低时,AMF的侵染率会更高。
本试验结果表明,AMF侵染显著促进南美蟛蜞菊的生长。类似的,Richard等[41]的研究表明, AMF侵染显著促进紫锥菊(Echinacea purpurea)的生长, 并且增加了其有价值的天然产物含量。Yuan等[42]和Zhang等[43]的研究表明,不同种类AMF对加拿大一枝黄花(Solidago canadensis)的影响不同。我们的研究表明,在难溶性磷处理下,接种AMF能显著提高叶片磷含量,其中GV可以形成更有效的共生关系。Majewska等[44]的研究表明,AMF促进了2种植物金光菊(Rudbeckia laciniata)和加拿大一枝黄花的生长和磷获取;Battini等[15]报道AMF和其表面细菌共同促进了植物生长和对磷的吸收,这与我们的研究结果一致。Li等[45]利用32P示踪量化研究表明,AMF有益于小麦(Triticum aestivum)从固磷土壤中吸收磷营养。
磷对植物生长至关重要,但也是植物最难获取的营养元素之一,尽管土壤中磷的总量通常超过植物需求,但大多数植物并不能直接吸收利用磷营养[46]。本研究中AMF促进了植株对难溶性磷的利用率。这可能是因为: (1)菌根共生增大了植物的营养可利用范围,AMF在土壤与根系中生长,可以形成一个发育良好的菌丝网络,增大吸收表面积[47],单个真菌菌丝的直径远小于根直径,可以进入更小的土壤孔隙,从而增加营养可利用范围[1, 48]。有研究表明磷酸盐离子在菌丝内扩散速度比根毛中更快,且菌丝吸收P的阈值浓度更低,从而使P快速转移到植株根部,加速磷在土壤中的扩散[49]。(2) AMF能分泌有机酸,还可以刺激寄主植物根部分泌有机酸促进难溶性磷的溶解,从而提高了磷的生物可利用性[18, 50]。有研究表明,菌根可以分泌释放草酸、柠檬酸等有机酸,这些有机酸可以溶解钙质磷酸盐, 如磷酸钙、羟基磷灰石,还可以使土壤中矿物表面结合的磷酸盐离子解析[51-52]。(3)分子遗传学研究表明,AMF能够诱导P转运蛋白基因的表达,使寄主植物产生更多的磷转运蛋白[53]。已有研究表明AMF的侵染促进了小麦[54]和马铃薯(Solanum tube-rosum)[55]中相关磷转运蛋白基因的表达。这种共生关系带来的优良营养吸收机制可能是南美蟛蜞菊资源利用效率高的因素之一,可能是其在占领新栖息地并能较好定殖过程中发挥了积极作用。
综上,在磷营养限制的情况下,GV和FM的侵染率更高,使得植物对难溶性磷的生物利用有所增加,从而更有益于南美蟛蜞菊的生长。在磷营养贫乏的环境中,AMF能促进南美蟛蜞菊对资源的获取和生长,这可能有助于其成功入侵更多的生境。未来可以利用近源的本地植物做对照,研究AMF对入侵植物与本地种的不同影响,从而明确AMF对植物入侵的贡献,更好地研究入侵机理,预测和管控外来植物入侵。
| [1] |
SMITH S E, READ D J. Mycorrhizal Symbiosis[M]. 3rd ed. London: Academic Press, 2008: 694.
|
| [2] |
TORREZ V, CEULEMANS T, MERGEAY J, et al. Effects of adding an arbuscular mycorrhizal fungi inoculum and of distance to donor sites on plant species recolonization following topsoil removal[J]. Appl Veg Sci, 2016, 19(1): 7-19. DOI:10.1111/avsc.12193 |
| [3] |
SCHÜBLER A, SCHWARZOTT D, WALKER C. A new fungal phylum, the Glomeromycota:Phylogeny and evolution[J]. Mycol Res, 2001, 105(12): 1413-1421. DOI:10.1017/s0953756201005196 |
| [4] |
FEDDERMANN N, FINLAY R, BOLLER T, et al. Functional diversity in arbuscular mycorrhiza:The role of gene expression, phosphorrous nutrition and symbiotic efficiency[J]. Fungal Ecol, 2010, 3(1): 1-8. DOI:10.1016/j.funeco.2009.07.003 |
| [5] |
AUGÉ R M, TOLER H D, SAXTON A M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions:A meta-analysis[J]. Mycorrhiza, 2015, 25(1): 13-24. DOI:10.1007/s00572-014-0585-4 |
| [6] |
FERROL N, TAMAYO E, VARGAS P. The heavy metal paradox in arbuscular mycorrhizas:from mechanisms to biotechnological appli-cations[J]. J Exp Bot, 2016, 67(22): 6253-6565. DOI:10.1093/jxb/erw403 |
| [7] |
SHRIVASTAVA G, OWNLEY B H, AUGÉ R M, et al. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect[J]. Symbiosis, 2015, 65(2): 65-74. DOI:10.1007/s13199-015-0319-1 |
| [8] |
HEIJDEN M G A, MARTIN F M, SELOSSE M A, et al. Mycorrhizal ecology and evolution:The past, the present, and the future[J]. New Phytol, 2015, 205(4): 1406-1423. DOI:10.1111/nph.13288 |
| [9] |
YANG H S, SCHROEDER-MORENO M, GIRI B, et al. Arbuscular mycorrhizal fungi and their responses to nutrient enrichment[M]// GIRI B, PRASAD R, VARMA A. Root Biology. Cham: Springer, 2018: 429-449. doi: 10.1007/978-3-319-75910-4_17.
|
| [10] |
ELSER J J. Phosphorus:a limiting nutrient for humanity?[J]. Curr Opin Biotechnol, 2012, 23(6): 833-838. DOI:10.1016/j.copbio.2012.03.001 |
| [11] |
ROBERTS T L, JOHNSTON A E. Phosphorus use efficiency and management in agriculture[J]. Resour Conserv Recy, 2015, 105: 275-281. DOI:10.1016/j.resconrec.2015.09.013 |
| [12] |
WANG H, APPAN A, GULLIVER J S. Modeling of phosphorus dynamics in aquatic sediments:Ⅱ. Examination of model performance[J]. Water Res, 2003, 37(16): 3939-3953. DOI:10.1016/S0043-1354(03)00305-1 |
| [13] |
SINDHU S S, PHOUR M, CHOUDHARY S R, et al. Phosphorus cycling: Prospects of using rhizosphere microorganisms for improving phosphorus nutrition of plants[M]//PARMAR N, SINGH A. Geomicrobiology and Biogeochemistry. Berlin, Heidelberg: Springer, 2014: 199-237. doi: 10.1007/978-3-642-41837-2_11.
|
| [14] |
OSORIO N W, HABTE M. Strategies for utilizing arbuscular mycorrhizal fungi and phosphate-solubilizing microorganisms for enhanced phosphate uptake and growth of plants in the soils of the tropics[M]// KHAN M S, ZAIDI A, MUSARRAT J. Microbial Strategies for Crop Improvement. Berlin: Springer, 2009: 325-351. doi: 10.1007/978-3-642-01979-1_16.
|
| [15] |
BATTINI F, GRØNLUND M, AGNOLUCCI M, et al. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria[J]. Sci Rep, 2017, 7(1): 4686. DOI:10.1038/s41598-017-04959-0 |
| [16] |
BAI Y F, GUO S X, LI M. Interactions between invasive plants and arbuscular mycorrhizal fungi:A review[J]. Chin J Appl Ecol, 2011, 22(9): 2457-2463. 柏艳芳, 郭绍霞, 李敏. 入侵植物与丛枝菌根真菌的相互作用[J]. 应用生态学报, 2011, 22(9): 2457-2463. DOI:10.13287/j.1001-9332.2011.0351 |
| [17] |
ZHANG L, XU M G, LIU Y, et al. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium[J]. New Phytol, 2016, 210(3): 1022-1032. DOI:10.1111/nph.13838 |
| [18] |
KOIDE R T, KABIR Z. Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate[J]. New Phytol, 2000, 148(3): 511-517. DOI:10.1046/j.1469-8137.2000.00776.x |
| [19] |
ZHANG L, FAN J Q, DING X D, et al. Hyphosphere interactions between an arbuscular mycorrhizal fungus and a phosphate solubilizing bacterium promote phytate mineralization in soil[J]. Soil Biol Biochem, 2014, 74: 177-183. DOI:10.1016/j.soilbio.2014.03.004 |
| [20] |
MALDONADO-MENDOZA I E, DEWBRE G R, HARRISON M J. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment[J]. Mol Plant Microbe Interact, 2001, 14(10): 1140-1148. DOI:10.1094/MPMI.2001.14.10.1140 |
| [21] |
PASZKOWSKI U, KROKEN S, ROUX C, et al. Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis[J]. Proc Natl Acad Sci USA, 2002, 99(20): 13324-13329. DOI:10.1073/pnas.202474599 |
| [22] |
XU G H, CHAGUE V, MELAMED-BESSUDO C, et al. Functional characterization of LePT4:A phosphate transporter in tomato with mycorrhiza-enhanced expression[J]. J Exp Bot, 2007, 58(10): 2491-2501. DOI:10.1093/jxb/erm096 |
| [23] |
FUMANAL B, PLENCHETTE C, CHAUVEL B, et al. Which role can arbuscular mycorrhizal fungi play in the facilitation of Ambrosia artemisiifolia L. invasion in France?[J]. Mycorrhiza, 2006, 17(1): 25-35. DOI:10.1007/s00572-006-0078-1 |
| [24] |
JI Y H, LIU W X, LIU R J, et al. Functions and mechanisms of arbuscular mycorrhizal fungi in succession of exotic invasive plants[J]. Plant Physiol J, 2013, 49(10): 973-980. 季彦华, 刘万学, 刘润进, 等. 丛枝菌根真菌在外来植物入侵演替中的作用与机制[J]. 植物生理学报, 2013, 49(10): 973-980. DOI:10.13592/j.cnki.ppj.2013.10.001 |
| [25] |
QI S S, DAI Z C, MIAO S L, et al. Light limitation and litter of an invasive clonal plant, Wedelia trilobata, inhibit its seedling recruitment[J]. Ann Bot, 2014, 114(2): 425-433. DOI:10.1093/aob/mcu075 |
| [26] |
SI C C, DAI Z C, LIN Y, et al. Local adaptation and phenotypic plasticity both occurred in Wedelia trilobata invasion across a tropical island[J]. Biol Invas, 2014, 16(11): 2323-2337. DOI:10.1007/s10530-014-0667-4 |
| [27] |
YU Z P, WANG M H, HUANG Z Q, et al. Temporal changes in soil C-N-P stoichiometry over the past 60 years across subtropical China[J]. Glob Change Biol, 2018, 24(3): 1308-1320. DOI:10.1111/gcb.13939 |
| [28] |
HE L P, KONG J J, LI G X, et al. Similar responses in morphology, growth, biomass allocation, and photosynthesis in invasive Wedelia trilobata and native congeners to CO2 enrichment[J]. Plant Ecol, 2018, 219(2): 145-157. DOI:10.1007/s11258-017-0784-0 |
| [29] |
MAJEWSKA M L, BŁASZKOWSKI J, NOBIS M, et al. Root-inhabiting fungi in alien plant species in relation to invasion status and soil chemical properties[J]. Symbiosis, 2015, 65(3): 101-115. DOI:10.1007/s13199-015-0324-4 |
| [30] |
WANG B, QIU Y L. Phylogenetic distribution and evolution of mycorrhizas in land plants[J]. Mycorrhiza, 2006, 16(5): 299-363. DOI:10.1007/s00572-005-0033-6 |
| [31] |
ZUBEK S, MAJEWSKA M L, BŁASZKOWSKI J, et al. Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils[J]. Biol Fert Soils, 2016, 52(6): 879-893. DOI:10.1007/s00374-016-1127-3 |
| [32] |
HU W W. Ecological effects of arbuscular mycorrhizal fungi (AMF) on common Compositae invasive species in Guangzhou[D]. Guangzhou: Sun Yat-sen University, 2015. 胡文武.丛枝菌根真菌(AMF)对广州常见菊科入侵植物的生态学效应研究[D].广州: 中山大学, 2015. http://cdmd.cnki.com.cn/Article/CDMD-10558-1015964283.htm |
| [33] |
ELBON A, WHALEN J K. Phosphorus supply to vegetable crops from arbuscular mycorrhizal fungi:A review[J]. Biol Agric Hort, 2015, 31(2): 73-90. DOI:10.1080/01448765.2014.966147 |
| [34] |
PÜSCHEL D, JANOUŠKOVÁ M, VOŘÍŠKOVÁ A, et al. Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp. through improved phosphorus acquisition[J]. Front Plant Sci, 2017, 8: 390. DOI:10.3389/fpls.2017.00390 |
| [35] |
PHILLIPS J M, HAYMAN D S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection[J]. Trans Br Mycol Soc, 1970, 55(1): 158-161. DOI:10.1016/s0007-1536(70)80110-3 |
| [36] |
TROUVELOT A, KOUGH J L, GIANINAZZI P V. Mesure du taux de mycorhization va d'un système radiculaire. Recherche de méthodes d'estimation ayant une signification fonctionnelle[M]// GIANINAZZI-PEARSON V, GIANINAZZI S. Physiological and Genetical Aspects of Mycorrhizae. Paris: INRA Press, 1986: 217-221.
|
| [37] |
WATTS-WILLIAMS S J, SMITH F A, JAKOBSEN I. Soil phosphorus availability is a driver of the responses of maize (Zea mays) to elevated CO2 concentration and arbuscular mycorrhizal colonisation[J]. Sym-biosis, 2019, 77(1): 73-82. DOI:10.1007/s13199-018-0573-0 |
| [38] |
BALZERGUE C, PUECH-PAGÈS V, BÉCARD G, et al. The regu-lation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events[J]. J Exp Bot, 2011, 62(3): 1049-1060. DOI:10.1093/jxb/erq335 |
| [39] |
ZALAMEA P C, TURNER B L, WINTER K, et al. Seedling growth responses to phosphorus reflect adult distribution patterns of tropical trees[J]. New Phytol, 2016, 212(2): 400-408. DOI:10.1111/nph.14045 |
| [40] |
WEN Z H, LI H B, SHEN Q, et al. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species[J]. New Phytol, 2019, 223(2): 882-895. DOI:10.1111/nph.15833 |
| [41] |
GUALANDI JR R J, AUGÉ R M, KOPSELL D A, et al. Fungal mutualists enhance growth and phytochemical content in Echinacea purpurea[J]. Symbiosis, 2014, 63(3): 111-121. DOI:10.1007/s13199-014-0293-z |
| [42] |
YUAN Y G, TANG J J, LENG D, et al. An invasive plant promotes its arbuscular mycorrhizal symbioses and competitiveness through its secondary metabolites:Indirect evidence from activated carbon[J]. PLoS One, 2014, 9(5): e97163. DOI:10.1371/journal.pone.0097163 |
| [43] |
ZHANG Q, YANG R Y, TANG J J, et al. Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion[J]. PLoS One, 2010, 5(8): e12380. DOI:10.1371/journal.pone.0012380 |
| [44] |
MAJEWSKA M L, ROLA K, ZUBEK S. The growth and phosphorus acquisition of invasive plants Rudbeckia laciniata and Solidago gigantea are enhanced by arbuscular mycorrhizal fungi[J]. Mycorrhiza, 2017, 27(2): 83-94. DOI:10.1007/s00572-016-0729-9 |
| [45] |
LI H Y, SMITH S E, HOLLOWAY R E, et al. Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus-fixing soil even in the absence of positive growth responses[J]. New Phytol, 2006, 172(3): 536-543. DOI:10.1111/j.1469-8137.2006.01846.x |
| [46] |
SMITH S E, JAKOBSEN I, GRØNLUND M, et al. Roles of arbuscular mycorrhizas in plant phosphorus nutrition:interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition[J]. Plant Physiol, 2011, 156(3): 1050-1057. DOI:10.1104/pp.111.174581 |
| [47] |
OLSSON P A, van AARLE I M, ALLAWAY W G, et al. Phosphorus effects on metabolic processes in monoxenic arbuscular mycorrhiza cultures[J]. Plant Physiol, 2002, 130(3): 1162-1171. DOI:10.1104/pp.009639 |
| [48] |
SCHNEPF A, LEITNER D, KLEPSCH S, et al. Modelling phosphorus dynamics in the soil-plant system[M]// BÜNEMANN E, OBERSON A, FROSSARD E. Phosphorus in Action. Berlin: Springer, 2011: 113-133. doi: 10.1007/978-3-642-15271-9_5.
|
| [49] |
BOLAN N S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants[J]. Plant Soil, 1991, 134(2): 189-207. DOI:10.1007/BF00012037 |
| [50] |
TAWARAYA K, NAITO M, WAGATSUMA T. Solubilization of insoluble inorganic phosphate by hyphal exudates of arbuscular mycorrhizal fungi[J]. J Plant Nutr, 2006, 29(4): 657-665. DOI:10.1080/01904160600564428 |
| [51] |
KIRK G J D. A model of phosphate solubilization by organic anion excretion from plant roots[J]. Eur J Soil Sci, 1999, 50(3): 369-378. DOI:10.1111/j.1365-2389.1999.00239.x |
| [52] |
AHONEN-JONNARTH U, van HEES P A W, LUNDSTRÖM U S, et al. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations[J]. New Phytol, 2000, 146(3): 557-567. DOI:10.1086/338034 |
| [53] |
KARANDASHOV V, BUCHER M. Symbiotic phosphate transport in arbuscular mycorrhizas[J]. Trends Plant Sci, 2005, 10(1): 22-29. DOI:10.1016/j.tplants.2004.12.003 |
| [54] |
GLASSOP D, SMITH S E, SMITH F W. Cereal phosphate transporters associated with the mycorrhizal pathway of phosphate uptake into roots[J]. Planta, 2005, 222(4): 688-698. DOI:10.1007/s00425-005-0015-0 |
| [55] |
RAUSCH C, DARAM P, BRUNNER S, et al. A phosphate transporter expressed in arbuscule-containing cells in potato[J]. Nature, 2001, 414(6862): 462-470. DOI:10.1038/35106601 |
 2020, Vol. 28
2020, Vol. 28



