2. 中国医药大学附设医院医学研究部, 中国台湾, 台中 40447
2. Department of Medical Research, China Medical University Hospital, China Medical University, Taichung 40441, Taiwang, China
植物内生真菌是指其全部或部分的生长发育过程在健康植物的茎干或叶片等组织中,而不引起明显病害症状的一类真菌[1]。研究表明植物内生真菌的次生代谢产物在抗肿瘤、促进宿主植物生长等多方面具有良好的作用[2-3],内生真菌次生代谢产物会表现出与宿主植物相同或者相似的生物活性。内生真菌和寄主植物之间的相互作用类型,受其共生体基因和外在环境调节的控制,其相互作用往往具有生长快速、抗逆境、抗病害等益处,比未感染植株更具生存竞争力[4]。在内生真菌-植物宿主-食草动物生态系统中,植物内生真菌扮演着前所未有的重要生态学作用。发挥这些作用的物质基础是内生真菌产生的丰富多样的次生代谢产物,它们具有多种生物活性。这些植物内生真菌与宿主之间特殊的共生关系及其自身独特的生物学特性为阐释药材道地性的形成带来了新的启发,同时也为从植物内生真菌次生代谢产物的研究中寻找新型的天然活性先导物质带来了新的方向。
前胡为伞形科(Umbelliferae)白花前胡(Peuce- danum praeruptorum)的干燥根,具有降气化痰、散风清热之功效。安徽宁国市是前胡道地产区之一, 所产前胡称为宁前胡[5]。前期从宁前胡中分离鉴定了一系列内生真菌[6],其中Fusarium tricinctum为宁前胡内生真菌中的优势菌株。不同发酵方式对内生真菌次级代谢产物产量、成分类别具有显著影响[7]。为探讨不同发酵方式对内生真菌优势菌株代谢产物的影响,分别对F. tricinctum采用固体和液体发酵法进行发酵,从液体发酵液中分离了13个单体化合物[8],本研究继续对其固体发酵的次生代谢产物进行研究。
1 材料和方法 1.1 材料AM-400、DRX 500和AVANCE III-600核磁共振(Bruker公司);Auto Premier P776质谱仪(Waters公司);制备液相(Agilent公司);中压制备(BÜCHI公司);柱色谱硅胶及薄层色谱硅胶板(青岛海洋化工厂);葡聚糖凝胶Sephadex LH-20 (GE公司)。
宁前胡采自安徽宁国(标本编号QHX34)。菌株从白花前胡(Peucedanum praeruptorum)新鲜根中分离得到,经形态及ITS rDNA鉴定为镰刀属Fusa- rium tricinctum (菌株编号LH10),菌株保存于安徽中医药大学药学院。
1.2 内生真菌的固体发酵培养500 mL锥形瓶中加入80 g蒸馏水清洗晾干大米,20 mL无机盐水溶液,100 mL蒸馏水。其中无机盐水溶液为:0.5% (NH4)2SO4、0.25% KH2PO4、0.1% MgSO4、0.1% NaCl。锥形瓶扎口,121℃灭菌30 min,按照10%的接种量接种于灭菌后的大米培养基中,28℃静置培养30 d。乙酸乙酯萃取,合并浓缩至蒸干得到固体发酵产物。
1.3 提取和分离乙酸乙酯物浸膏55 g经硅胶柱层析,以石油醚-丙酮系统梯度洗脱(1:0~0:1, V/V)得7个组分A~ G。组分C经中压色谱以甲醇-水(10:100~100:0)梯度洗脱得到6个组分,组分C-5经Sephadex LH-20 (氯仿-甲醇)洗脱得到3个组分,C-5-1再经制备液相(乙腈/水, 70:30→100:0)制备得化合物11 (14.2 mg, 36.3 min)和10 (15.4 mg, 37.5 min)。组分C-5-3经制备液相(乙腈/水, 70:30→100:0)制备得化合物9 (34 mg, 38.2 min)和12 (6.2 mg, 39.6 min)。将C-6经Sephadex LH-20 (氯仿/甲醇)柱洗脱得到组分C-6-1, 将C-6-1再经制备液相(乙腈/水, 70:30→100:0)制备得化合物14 (9.5 mg, 33.2 min)和13 (10.3 mg, 32.0 min)。组分D经硅胶柱层析,以石油醚-丙酮(15:1~5:1)洗脱得到3个组分,组分D-2经中压色谱以甲醇-水(10:100~100:0)梯度洗脱得到5个组分,组分D-2-2经Sephadex LH-20用纯甲醇洗脱,再经制备液相(乙腈/水, 10%~25%)得到化合物1 (19.5 mg, 28.5 min)和2 (7.5 mg, 26.4 min)。部位E经中压色谱以甲醇-水(10:100~100:0)梯度洗脱得到6个组分,组分E-1经Sephadex LH-20 (甲醇)柱洗脱,组分E-1-3再经制备液相(乙腈/水, 10:90→25:75)制备得化合物5 (6.5 mg, 26.6 min)和6 (4.4 mg, 27.5 min)。组分E-2经Sephadex LH-20用纯甲醇洗脱,E-2-2再经制备液相(乙腈/水, 10:90→25:75))制备得化合物3 (8.5 mg, 25.6 min)和4 (7.4 mg, 23.3 min)。组分F经中压色谱以甲醇-水(10:100~100:0)梯度洗脱得到4个组分,组分F-2经Sephadex LH-20 (甲醇)柱洗脱,再将组分F-2-2经制备液相(乙腈/水, 10%~25%)得化合物7 (4.3 mg, 33.4 min)和8 (3.5 mg, 27.2 min)。
1.4 结构鉴定化合物1 针状结晶。ES I-MS m/z: 235 [M + H]+,易溶于甲醇。1H NMR (CD3OD, 400 MHz): δ 7.40 (1H, s, H-6), 7.38 (1H, d, J = 15.6 Hz, H-3), 6.18 (1H, d, J = 15.2 Hz, H-2), 4.88 (1H, t, J = 7.0 Hz, H- 10), 3.53 (3H, s, H-15), 3.39 (3H, s, H-14), 3.27 (2H, dd, J = 6.8, 13.6 Hz, H-9), 1.58 (3H, s, H-12), 1.52 (3H, s, H-13); 13C NMR (CD3OD, 125 MHz): δ 169.9 (C-1), 140.4 (C-6), 136.4 (C-3), 135.6 (C-8), 134.2 (C-4), 120.3 (C-10), 114.6 (C-2), 51.9 (C-15), 32.1 (C-14), 25.7 (C-12), 23.0 (C-9), 18.0 (C-13)。根据文献[9-10]报道,确定化合物1为fungerin。
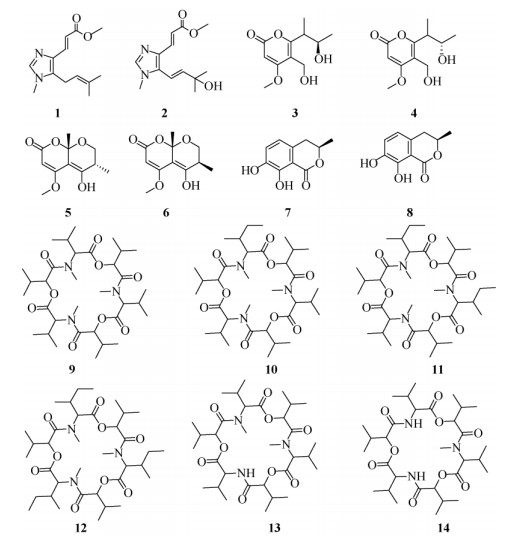
|
图 1 化合物1~14的结构 Fig. 1 Structures of compounds 1-14 |
化合物2 针状结晶。ES I-MS m/z: 273 [M + Na]+,易溶于甲醇。1H NMR (CD3OD, 400 MHz): δ 7.70 (1H, d, J = 15.3 Hz, H-3), 7.66 (1H, s, H-6), 6.60 (1H, d, J = 16.0 Hz, H-9), 6.51 (1H, d, J = 15.6 Hz, H-2), 6.27 (1H, d, J = 16.4 Hz, H-10), 3.77 (3H, s, H- 15), 3.70 (3H, s, H-14), 1.43 (3H, s, H-12), 1.41 (3H, s, H-13); 13C NMR (CD3OD, 125 MHz): δ 169.8 (C- 1), 147.3 (C-10), 141.0 (C-6), 136.6 (C-3), 135.2 (C-4), 134.4 (C-8), 115.8 (C-2), 112.5 (C-9), 71.9 (C-11), 52.0 (C-15), 32.8 (C-14), 29.8 (C-12), 29.8 (C-13)。根据文献[9-10]报道,确定化合物2为fusagerins F。
化合物3 白色粉末。ES I-MS m/z: 251 [M + Na]+,易溶于甲醇。1H NMR (CD3OD, 400 MHz): δ 5.58 (1H, s, H-3), 4.45 (2H, s, H-5a), 3.92 (3H, s, OCH3), 3.65 (1H, m, H-8), 3.02 (1H, m, H-7), 1.27 (3H, d, J = 6.4 Hz, H-8a), 1.19 (3H, d, J = 7.2 Hz, H- 7a); 13C NMR (CD3OD, 125 MHz): δ 172.6 (C-4), 167.6 (C-2), 166.9 (C-6), 113.7 (C-5), 88.4 (C-3), 70.2 (C-8), 57.2 (OCH3), 54.0 (C-5a), 44.0 (C-7), 21.5 (C- 8a), 15.2 (C-7a)。根据文献[11]报道,确定化合物3为6-[(3R)-3-hydroxybutan-2-yl]-5-(hydroxymethyl)-4- methoxy-2H-pyran-2-one
化合物4 白色粉末。ESI-MS m/z: 251 [M + Na]+,易溶于甲醇。1H NMR (CD3OD, 400 MHz): δ 5.58 (1H, s, H-3), 4.45 (2H, s, H-5a), 3.92 (3H, s, OCH3), 3.53 (1H, m, H-8), 3.02 (1H, m, H-7), 1.27 (3H, d, J = 6.4 Hz, H-8a), 1.19 (3H, d, J = 7.2 Hz, H- 7a); 13C NMR (CD3OD, 125 MHz): δ 172.6 (C-4), 167.6 (C-2), 166.9 (C-6), 113.7 (C-5), 88.4 (C-3), 68.7 (C-8), 57.2 (OCH3), 54.1 (C-5a), 43.5 (C-7), 21.2 (C- 8a), 15.6 (C-7a)。根据文献[11]报道,确定化合物4为6-[(3S)-3-hydroxybutan-2-yl]-5-(hydroxymethyl)-4- methoxy-2H-pyran-2-one。
化合物5 白色粉末。ES I-MS m/z: 249 [M + Na]+,易溶于甲醇。1H NMR (CD3OD, 400 MHz): δ 5.59 (1H, s, H-3), 4.51 (1H, dd, J = 3.2, 11.6 Hz, H- 7a), 4.40 (1H, dd, J = 5.2, 8.0 Hz, H-7b), 3.89 (3H, s, OCH3), 2.80 (1H, m, H-6), 1.46 (3H, d, J = 7.4 Hz, H- 8), 1.26 (3H, s, H-9); 13C NMR (CD3OD, 125 MHz): δ 170.8 (C-2), 167.2 (C-5), 160.9 (C-4), 107.3 (C-4a), 97.8 (C-8a), 88.1 (C-3), 56.9 (C-7), 56.5 (OCH3), 41.7 (C-6), 26.5 (C-8), 15.9 (C-9)。根据文献[12]报道,确定化合物5为6α-chlamydosporol。
化合物6 白色粉末。ES I-MS m/z: 249 [M + Na]+,易溶于甲醇。1H NMR (400 MHz, CD3OD): δ 5.59 (1H, s, H-3), 4.51 (1H, dd, J = 3.2, 11.6 Hz, H- 7a), 4.40 (1H, dd, J = 5.2, 8.0 Hz, H-7b), 3.89 (3H, s, OCH3), 2.80 (1H, m, H-6), 1.46 (3H, d, J = 7.4 Hz, H- 8), 1.26 (3H, s, H-9); 13C NMR (125 MHz, CD3OD): δ 170.8 (C-2), 166.8 (C-5), 159.5 (C-4), 108.1 (C-4a), 98.2 (C-8a), 88.4 (C-3), 57.1 (C-7), 56.5 (OCH3), 41.5 (C-6), 25.6 (C-8), 11.4 (C-9)。根据文献[12]报道,确定化合物6为6β-chlamydosporol。
化合物7 白色粉末。ES I-MS m/z: 217 [M + Na]+,易溶于甲醇氯仿。1H NMR (400 MHz, CDCl3): δ 11.0 (1H, s, H-9a), 7.54 (1H, t, J = 7.6 Hz, H-7), 7.03 (1H, d, J = 8.4 Hz, H-6), 6.93 (1H, d, J = 7.2 Hz, H-8), 4.84 (1H, d, J = 5.6 Hz, H-4), 4.7 (1H, m, H-3), 1.59 (3H, d, J = 6.8 Hz, H-3a); 13C NMR (125 MHz, CDCl3): δ 169.2 (C-1), 162.0 (C-9), 140.5 (C-5), 136.7 (C-7), 118.5 (C-6), 118.4 (C-8), 118.3 (C-10), 78.2 (C-3), 67.2 (C-4), 16.0 (C-3a)。根据文献[13]报道,确定化合物7为(3R, 4R)-(-)-4-hydroxymellein。
化合物8 白色粉末。ESI-MS m/z: 217 [M + Na]+,易溶于甲醇、氯仿。1H NMR (400 MHz, CDCl3): δ 10.58 (1H, s, H-8), 7.01 (1H, d, J = 8.8 Hz, H-7), 6.78 (1H, d, J = 8.8 Hz, H-6), 5.12 (1H, m, H-3), 3.17~3.12 (1H, dd, J = 3.2, 13.6 Hz, H-4a), 2.71~2.64 (1H, m, H- 4b), 1.38 (3H, d, J = 7.2 Hz, H-11); 13C NMR (150 MHz, CDCl3): δ 170.1 (C-1), 156.4 (C-9), 143.9 (C-8), 124.6 (C-5), 124.2 (C-6), 116.2 (C-7), 108.5 (C-10), 76.2 (C- 3), 28.7 (C-4), 21.1 (C-11)。根据文献[14]报道,确定化合物8为(3R)-(-)-8-hydroxymellein。
化合物9 白色粉末。ES I-MS m/z: 662 [M + Na]+,易溶于氯仿。1H NMR (400 MHz, CDCl3): δ 5.21 (1H, d, J = 8.0 Hz, H-6), 4.72 (1H, t, J = 8.8 Hz, H-3), 3.26 (3H, s, H-4a), 2.35~2.29 (1H, m, H-10), 2.23~2.16 (1H, m, H-7), 1.11 (3H, d, J = 6.8 Hz, H-11, H-12), 1.05 (3H, d, J = 6.4 Hz, H-8, H-9); 13C NMR (125 MHz, CDCl3): δ 172.8 (C-3), 172.2 (C-5), 76.9 (C-6), 63.7 (C-3), 33.1 (C-4a), 31.6 (C-7), 29.5 (C-10), 20.8 (C-11), 20.7 (C-12), 19.1 (C-8), 18.7 (C-9)。根据文献[15]报道,确定化合物9为enniatin B。
化合物10 白色结晶。ES I-MS m/z: 676 [M + Na]+,易溶于氯仿。1H NMR (400 MHz, CDCl3): δ 5.28 (1H, t, J = 7.2 Hz, H-5, H-11), 4.70 (1H, t, J = 8.4 Hz, H-2, H-8), 3.23 (3H, s, H-1a, H-7a), 3.10~ 3.06 (1H, m, H-23), 2.35~2.27 (1H, m, H-16), 2.21~ 2.10 (1H, m, H-13, H-20), 1.60~1.48 (2H, m, H-18), 1.12 (3H, d, J = 6.8 Hz, H-17), 1.05 (3H, d, J = 6.4 Hz, H-19), 0.96 (3H, d, J = 6.4 Hz, H-24, H-25), 0.90 (3H, d, J = 6.4 Hz, H-21, H-22), 0.88 (3H, d, J = 6.4 Hz, H- 14, H-15); 13C NMR (125 MHz, CDCl3): δ 172.8 (C- 3), 172.7 (C-9), 172.2 (C-6), 172.1 (C-12), 76.9 (C-5), 76.7 (C-11), 63.7 (C-2), 61.8 (C-8), 35.6 (C-16), 33.0 (C-1a, 7a), 31.6 (C-13, 20), 29.5 (C-23), 27.4 (C-18), 20.8 (C-24, 25), 19.0 (C-21, 22), 18.7 (C-14, 15), 16.8 (C-17), 11.3 (C-19)。根据文献[15]报道,确定化合物10为enniatin B1。
化合物11 白色结晶。ES I-MS m/z: 690 [M + Na]+,易溶于氯仿。1H NMR (400 MHz, CDCl3): δ 5.22 (1H, t, J = 7.2 Hz, H-5, H-11), 4.70 (1H, t, J = 8.8 Hz, H-2, H-8), 3.23 (3H, s, H-1a, H-7a), 3.11~ 3.06 (1H, m, H-23), 2.35~2.27 (1H, m, H-16), 2.22~ 2.10 (1H, m, H-13, H-20), 1.60-1.48 (2H, m, H-18), 1.12 (3H, d, J = 6.8 Hz, H-17), 1.05 (3H, d, J = 6.4 Hz, H-19), 0.96 (3H, d, J = 6.4 Hz, H-24, H-25), 0.90 (3H, d, J = 6.4 Hz, H-21, H-22), 0.88 (3H, d, J = 6.4 Hz, H- 14, H-15); 13C NMR (125 MHz, CDCl3): δ 172.8 (C- 3), 172.7 (C-9), 172.2 (C-6), 172.1 (C-12), 76.9 (C-5), 76.7 (C-11), 63.7 (C-2), 61.8 (C-8), 35.6 (C-16), 33.0 (C-1a, 7a), 31.6 (C-13, 20), 29.5 (C-23), 27.4 (C-18), 20.8 (C-24, 25), 19.0 (C-21, 22), 18.7 (C-14, 15), 16.8 (C-17), 11.3 (C-19)。根据文献[15]报道,确定化合物11为enniatin A1。
化合物12 白色粉末。ESI-MS m/z: 704 [M + Na]+,易溶于氯仿。1H NMR (400 MHz, CDCl3): δ 5.23 (1H, d, J = 8.0 Hz, H-6), 4.74 (1H, d, J = 8.8 Hz, H-3), 3.23 (3H, s, H-4a), 2.34~2.30 (1H, m, H-10), 2.26~2.19 (1H, m, H-7), 1.10 (3H, d, J = 6.4 Hz, H-11), 1.04 (3H, t, J = 6.4 Hz, H-13), 0.94 (3H, t, J = 6.4 Hz, H-8, H-9); 13C NMR (125 MHz, CDCl3): δ 172.0 (C- 2), 171.7 (C-5), 76.5 (C-6), 61.9 (C-3), 33.2 (C-4a), 31.4 (C-10), 29.4 (C-7), 27.0 (C-12), 20.6 (C-8, 9), 16.6 (C-11), 11.0 (C-13)。根据文献[16]报道,确定化合物12为enniatin A。
化合物13 白色结晶。ES I-MS m/z: 634 [M + Na]+,易溶于氯仿。1H NMR (400 MHz, CDCl3): δ 8.10 (1H, s, H-1), 5.14 (1H, d, J = 5.6 Hz, H-5), 4.62 (1H, d, J = 8.4 Hz, H-2), 4.43 (1H, d, J = 10.0 Hz, H- 8), 3.16 (3H, s, H-7a), 2.98 (1H, m, H-9), 2.58~2.47 (1H, m, H-12), 2.22~2.04 (1H, m, H-15), 1.03 (3H, d, J = 6.8 Hz, H-16, H-17), 0.88 (3H, d, J = 6.4 Hz, H-10, H-11), 0.85 (3H, d, J = 6.4 Hz, H-13, H-14); 13C NMR (125 MHz, CDCl3): δ 173.5 (C-3), 172.2 (C-6), 77.0 (C-5), 76.6 (C-8), 64.2 (C-2), 32.5 (C-7a), 31.6 (C-15), 29.4 (C-12), 29.0 (C-9), 21.5 (C-10, 11), 20.6 (C-16, 17), 18.8 (C-13, 14)。根据文献[17]报道,确定化合物13为enniatin B3。
化合物14 白色结晶。ESI-MS m/z: 648 [M + Na]+,易溶于氯仿。1H NMR (400 MHz, CDCl3): δ 8.21 (1H, s, H-1), 5.18 (1H, d, J = 5.6 Hz, H-5), 4.56 (1H, d, J = 8.4 Hz, H-2), 4.39 (1H, d, J = 10.0 Hz, H- 8), 3.18 (3H, s, H-7a), 3.02 (1H, m, H-9), 2.61~2.52 (1H, m, H-12), 2.22~2.04 (1H, m, H-15), 1.01 (3H, d, J = 6.8 Hz, H-16, H-17), 0.94 (3H, d, J = 6.4 Hz, H-10, H-11), 0.88 (3H, d, J = 6.4 Hz, H-13, H-14); 13C NMR (125 MHz, CDCl3): δ 173.7 (C-3), 172.1 (C-6), 76.9 (C-5), 76.3 (C-8), 63.9 (C-2), 32.3 (C-7a), 29.6 (C-15), 29.4 (C-12), 29.0 (C-9), 21.5 (C-10, 11), 20.4 (C-16, 17), 18.6 (C-13, 14)。根据文献[17]报道,确定化合物14为enniatin B2。
1.5 抗肿瘤活性结果将化合物对5种口腔癌细胞株CAR、CAL27、SCC-4、SCC-9、HSC-3进行抗肿瘤活性筛选,结果表明,化合物均无显著活性。
2 结果和讨论前期研究从宁前胡中分离鉴定了一系列内生真菌,Fusarium tricinctum为宁前胡内生真菌中的优势菌株,其隶属于瘤座孢科(Discellaceae)镰刀属[18], 是一类全世界广泛分布的真菌,Fusarium tricinctum作为一类重要的真菌资源,表现出多样的价值,越来越受到重视。其次生代谢产物往往具有良好的生理活性。Zhang等从F. tricinctum中分离得到了1个独特骨架的新的倍半萜类化合物fusartricin, 对Enterobacter aerogenes、Micrococcus tetragenu和Candida albicans具有显著的抗菌活性[19]。Sun等从F. tricinctum SYPF 7082中分离得到2个新的生物碱, 其中[-(α-oxyisohexanoyl-N-methyl-leucyl)2-]对小鼠巨噬细胞产生一氧化氮有显著的抑制作用[20]。
前期从宁前胡内生菌F. tricinctum液体发酵液中分离鉴定了13个化合物,分别为cyclo-(L-pro-L-pro)、cyclo-(S-pro-S-leu)、cyclo-(L-phe-L-phe)、cyclo-(D-pro- L-phe)、cyclo-(L-pro-L-phe)、cyclo-(D-pro-L-leu)、cyclo- (S-pro-S-leu)、cyclo-[D-(4-hydroxyprolinyl)]-(L)-leucine、cyclo-[L-(4-hydroxyprolinyl)]-(L)-leucine、cyclo-(trans- 4-hydroxy-L-prolyl-L-phenylalanine)、cyclo-(D-cis-hyp- L-phe)、苯甲酸苄酯和苯乙酸。体外抗肿瘤活性试验表明3个化合物对CAR、CAL27、SCC-4、SCC-9和HSC-3等5种口腔癌肿瘤细胞有一定的细胞毒活性。为比较不同发酵方式对内生真菌优势菌株代谢产物的影响,分别对宁前胡内生真菌F. tricinctum进行固体和液体发酵法比较研究,结果表明不同发酵方式的宁前胡优势菌株发酵产物完全不同。本研究在该菌株液体发酵次生代谢产物研究基础上,从F. tricinctum固体发酵液中分离并鉴定了14个单体化合物。所有化合物均为首次从宁前胡内生真菌中分离得到,且同菌株不同发酵方式的发酵液中没有结构相同的次生代谢产物,尤其在固体发酵代谢产物中分离的环肽类化合物在液体发酵中并未分离到,说明发酵方式对代谢产物结构有重要影响。已有报道的化合物Enniatins A1、B和B1能够产生抗癌活性[21],但本研究的所有化合物对5种口腔癌肿瘤细胞均无显著的细胞毒活性。目前国内外对宁前胡内生菌的次生代谢产物研究无相关报道,本研究进一步丰富了宁前胡内生真菌F. tricinctum次生代谢产物结构多样性。
| [1] |
RODRIGUEZ R J, WHITE J F Jr, ARNOLD A E, et al. Fungal endophytes:Diversity and functional roles[J]. New Phytol, 2009, 182(2): 314-330. DOI:10.1111/j.1469-8137.2009.02773.x |
| [2] |
UZMA F, MOHAN C D, HASHEM A, et al. Endophytic fungi-alter-native sources of cytotoxic compounds:A review[J]. Front Pharmacol, 2018, 26(9): 309. DOI:10.3389/fphar.2018.00309 |
| [3] |
DESHMUKH S K, VEREKAR S A. Fungal endophytes:A potential source of antifungal compounds[J]. Front Biosci, 2012, 4: 2045-2070. DOI:10.2741/E524 |
| [4] |
MORICCA S, RAGAZZI A. Fungal endophytes in Mediterranean oak forests:A lesson from Discula quercina[J]. Phytopathology, 2008, 98(4): 380-386. DOI:10.1094/phyto-98-4-0380 |
| [5] |
QIU X X, ZHANG L, YUE J Y, et al. Investigation on the factors affecting the contents of three coumarins in Ningguo Peucedanum praeruptorum Dunn[J]. J Chin Med Mat, 2016, 39(4): 713-716. 邱晓霞, 张玲, 岳婧怡, 等. 宁前胡中3种香豆素含量影响因素的考察[J]. 中药材, 2016, 39(4): 713-716. DOI:10.13863/j.issn1001-4454.2016.04.004 |
| [6] |
LIU H T, SHAO J, WANG G, et al. Culture/isolation and identification of endophytic fungi of Ningguo Peucedanum praeruptorum Dunn[J]. J Anhui Univ Chin Med, 2018, 37(4): 87-91. 刘海涛, 邵杰, 王刚, 等. 宁前胡内生真菌的培养分离与鉴定[J]. 安徽中医药大学学报, 2018, 37(4): 87-92. DOI:10.3969/j.issn.2095-7246.2018.04.023 |
| [7] |
LIN S T, LI C F, HU X P, et al. Effects of different fermentation methods on secondary metabolites synthesis by endophytical fungi CH1307c from Cephalotaxus hainanensis Li[J]. Chin J Trop Crops, 2016, 37(7): 1407-1412. 林淑婷, 李从发, 胡晓苹, 等. 不同发酵方式对海南粗榧内生真菌CH1307c合成次级代谢产物的影响[J]. 热带作物学报, 2016, 37(7): 1407-1412. DOI:10.3969/j.issn.1000-2561.2016.07.026 |
| [8] |
LIU C B, LIU H T, YANG J X, et al. Secondary metabolites of endophytic fungus Fusarium tricinctum from Ningguo Peucedanum praeruptorum Dunn[J]. Nat Prod Res Dev, 2019, 31(9): 1580-1584. 刘丛彬, 刘海涛, 杨家欣, 等. 宁前胡内生真菌Fusarium tricinctum次生代谢产物研究[J]. 天然产物研究与开发, 2019, 31(9): 1580-1584. DOI:10.16333/j.1001-6880.2019.9.014 |
| [9] |
WEN H, LI Y, LIU X Z, et al. Fusagerins A-F, new alkaloids from the fungus Fusarium sp.[J]. Nat Prod Bioprospect, 2015, 5(4): 195-203. DOI:10.1007/s13659-015-0067-1 |
| [10] |
PRZYBYLA D, NUBBEMEYER U. 4, 5-disubstituted N-methylimidazoles as versatile building blocks for defined side-chain introduction[J]. Eur J Org Chem, 2017, 2017(3): 695-703. DOI:10.1002/ejoc.201601384 |
| [11] |
SOLFRIZZO M, VISCONTI A, SAVARD M E, et al. Isolation and characterization of new chlamydosporol related metabolites of Fusarium chlamydosporum and F. tricinctum[J]. Mycopathologia, 1994, 127(2): 95-101. DOI:10.1007/BF01103065 |
| [12] |
SAVARD M E, MILLER J D, SALLEH B, et al. Chlamydosporol, a new metabolite from Fusarium chlamydosporum[J]. Mycopathologia, 1990, 110(3): 177-181. DOI:10.1007/BF00437543 |
| [13] |
TAKESUE T, FUJITA M, SUGIMURA T, et al. A series of two oxidation reactions of ortho-alkenylbenzamide with hypervalent iodine (III):A concise entry into (3R, 4R)-4-hydroxymellein and (3R, 4R)-4-hydroxy-6-methoxymellein[J]. Org Lett, 2014, 16(17): 4634-4637. DOI:10.1021/ol502225p |
| [14] |
DEVYS M, BARBIER M, BOUSQUET J F, et al. Isolation of the (2R)-(3R)-5-hydroxymellein from the fungus Septoria nodorum[J]. Phytochemistry, 1994, 35(3): 825-826. DOI:10.1016/s0031-9422(00)90617-4 |
| [15] |
JI Z Q, WU W J, WANG M A, et al. Identification of fungicidal compounds from endophytic fungi Fusarium proliferatum in Celastrus angulatus[J]. J NW Sci Techn Univ Agric For (Nat Sci), 2005, 33(5): 61-64. 姬志勤, 吴文君, 王明安, 等. 苦皮藤内生真菌层出镰刀菌中杀菌成分的结构鉴定[J]. 西北农林科技大学学报(自然科学版), 2005, 33(5): 61-64. DOI:10.13207/j.cnki.jnwafu.2005.05.015 |
| [16] |
TSANTRIZOS Y S, XU X J, SAURIOL F, et al. Novel quinazolinones and enniatins from Fusarium lateritium Nees[J]. Can J Chem, 1993, 71(9): 1362-1367. DOI:10.1139/v93-176 |
| [17] |
VISCONTI A, BLAIS L A, APSIMON J W, et al. Production of enniatins by Fusarium acuminatum and Fusarium compactum in liquid culture:Isolation and characterization of three new enniatins, B2, B3, and B4[J]. J Agric Food Chem, 1992, 40(6): 1076-1082. DOI:10.1021/jf00018a034 |
| [18] |
WEI J C. Handbook of Fungal Identification[M]. Shanghai: Shanghai Scientific & Technical Publishers, 1979: 609. 魏景超. 真菌鉴定手册[M]. 上海: 上海科学技术出版社, 1979: 609. |
| [19] |
ZHANG J N, LIU D, WANG H, et al. Fusartricin, a sesquiterpenoid ether produced by an endophytic fungus Fusarium tricinctum Salicorn 19[J]. Eur Food Res Technol, 2015, 240(4): 805-814. DOI:10.1007/s00217-014-2386-6 |
| [20] |
SUN W J, ZHU H T, ZHANG T Y, et al. Two new alkaloids from Fusarium tricinctum SYPF 7082, an endophyte from the root of Panax notoginseng[J]. Nat Prod Bioprospect, 2018, 8(5): 391-396. DOI:10.1007/s13659-018-0171-0 |
| [21] |
WÄTJEN W, DEBBAB A, HOHLFELD A, et al. Enniatins A1, B and B1 from an endophytic strain of Fusarium tricinctum induce apoptotic cell death in H4IIE hepatoma cells accompanied by inhibition of ERK phosphorylation[J]. Mol Nutri Food Res, 2009, 53(4): 431-440. DOI:10.1002/mnfr.200700428 |
 2020, Vol. 28
2020, Vol. 28


