2. 中国科学院华南植物园, 广东省应用植物学重点实验室, 广州 510650
2. Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China
链霉菌是一类革兰氏阳性菌,19世纪末,研究者从土壤中分离出大量好气腐生菌,1943年将这类菌归为链霉菌属(Streptomyces sp.)[1]。链霉菌属于原核生物界、放线菌目(Actinobacterales)、链霉菌科(Streptomycetaceae)。链霉菌是放线菌目中最大的一个属,包含1 000多种菌。链霉菌从孢子萌发到孢子释放完成整个生长周期。孢子在固体培养基上萌发, 逐步产生基内菌丝,基内菌丝多核而有分支, 吸收营养,进行营养生长。营养生长阶段后期,基内菌丝不断降解,代谢出不同色素,形成不同颜色。培养基表面产生气生菌丝,气生菌丝具有疏水性,在气生菌丝上继而形成孢子丝,孢子丝是由单核的孢子构成。孢子再萌发从而构成链霉菌的一个生长周期[2]。在链霉菌分类系统中,孢子链与孢子形态是重要的参考依据。链霉菌基因组染色体呈线状,结构复杂,是目前已知最大的原核生物基因组。
链霉菌具有多种功能,有些能够产生抗生素与植物生长激素类物质,促进植物生长,提高植物对于生物与非生物逆境的抵御能力[3]。目前已知的链霉菌产生的9 000多种具有生物活性的物质中,超过120多种物质得到了应用,占微生物来源的生物活性物质应用品种数量的75%[4]。目前已知抗生素种类有16 500种,其中51%是由链霉菌产生。链霉菌分泌的农用抗生素作为低毒、低残留的生物农药逐步受到人们的关注。
微生物与植物之间通过互相作用各取所需。微生物通过在植物根部附近吸收植物分泌的营养物质,进行生长繁殖,而植物则摄取微生物的代谢物质促进自身生长。链霉菌作为一类具有多种功能的微生物,在农业生产活动中得到了广泛的应用。本文对国内外有关链霉菌在植物生长上的功能研究进行综述,并展望其应用前景和将来的研究方向, 为进一步发挥其生物防治作用,开发出环境友好型的低毒、高效与高产的抗生素提高帮助,从而更好地服务于国家生态文明建设。
1 链霉菌链霉菌是细菌界、放线菌目中的一类细菌。大部分链霉菌具有菌丝与孢子,少数链霉菌在多次继代培养过程中会丧失产生孢子能力。链霉菌定殖的植物种类广泛,如在棉花(Gossypium spp.)、黄瓜(Cucumis sativus)、毛白杨(Populus tomentosa)、黄花蒿(Artemisia annua)、水稻(Oryza sativa)、大麦(Hordeum vulgare)和花生(Arachis hypogaea)等30多种植物上都可以定殖,定殖部位多,从根、茎、叶片中都有链霉菌分离出来(表 1)。有多种技术手段可以研究链霉菌在植物中的定殖情况。如携带绿色荧光蛋白基因的生防链霉菌株SSD49-pIJ8660Ep能够定殖到毛白杨组培苗的茎和叶片中,当接种SSD49- pIJ8660Ep后,在茎段切片中可以看到明显的绿色荧光,在叶片中也能看到散布的微弱绿色荧光,但根中并未观察到绿色荧光。表明链霉菌沿着伤口进入植物体内,在茎中分布较多,随后迁移至叶片中, 但链霉菌难以进入根部[5]。壮观链霉菌(S. spectabilis)在棉花根际土壤和根部长期定殖,30 d的定殖密度分别为1.38×105和2.95×103 CFU g-1[6]。谯天敏等[7]利用实时荧光定量PCR技术监测铁核桃(Juglans sigllata)根中绛红褐链霉菌(S. purpeofuscus)特异性基因表达量,结果表明其呈先增大后降低的趋势,40 d后稳定。
| 表 1 链霉菌在植物生长和生物防治中的功能 Table 1 Effects of Streptomyces on plant growth and biological control |
在室内培养时,不同的链霉菌在同种培养基上会有不同的形态特征,这也是早期链霉菌归类的主要依据。目前链霉菌分类主要依靠表观分类、数值分类、化学分类、分子分类与多相分类,从早期人为因素多、试验结果重复性差逐步向着更加客观与标准的方向发展。20世纪50~60年代,链霉菌分类主要依靠链霉菌孢子链的形态、孢子大小、孢子表面特征、气生菌丝、基内菌丝的颜色,是否产生可溶性色素、是否产生黑色素、对不同碳氮源的利用和对抗生素的敏感性等指标[8]。20世纪70年代开始,Lechevalier[9]将细胞壁化学组分分析应用于细菌分类,提出将形态学分类与化学分类相结合的方法来划分属,链霉菌属细胞壁类型为Ⅰ型。20世纪80年代,分子生物学技术开始应用于放线菌的分类,Woese等[10]利用16S rDNA序列相似性,采用DNA-rRNA和DNA-DNA杂交等技术建立了放线菌的系统进化树。目前,链霉菌分类通常采用多相分类的方法,将表观分类、化学分类和分子分类综合起来进行分析。依据链霉菌的生存环境不同,可以将链霉菌初步分为土壤链霉菌、海洋链霉菌与植物内生链霉菌。
大部分链霉菌对植物具有促进生长、增强营养吸收、提高抵御生物与非生物逆境等有益作用[11-12], 但也有研究表明某些链霉菌具有病原菌的特性,如由链霉菌引起的马铃薯(Solanum tuberosum)疮痂病[13], 马铃薯品种对于土壤中链霉菌的丰度具有显著影响,感病植株周围存在大量链霉菌,而抗病植株附近链霉菌较少存在。链霉菌还可以提高其他微生物在植物根部的定殖能力,Nadine等[14]研究表明链霉菌AcH 505可以提高担子菌331在云杉(Picea asperata)根部的定殖率,链霉菌能诱导担子菌331体内水杨酸类似物的分泌。对链霉菌这些正面与负面影响的研究,为后续病原菌针对性防控奠定了一定的基础。
2 链霉菌促进植物生长,增强营养吸收研究表明,链霉菌主要通过分泌植物生长调节剂如IAA、嗜铁素等发挥促进植物生长的作用[15]。
利迪链霉菌(S. lydicus)能促进西红柿(Lycopersicon esculentum)生长,增加叶片数目,增强光合作用, 西红柿体内脱落酸、茉莉酸与水杨酸等植物生长调节剂含量都有增加[16]。链霉菌RP1A-12能够促进花生种子发芽,提高茎与芽的长度[17]。温室与大田试验表明,链霉菌C2012与C801可以提高薄荷(Mentha canadensis)地上部分的鲜质量与干质量[18]。分离自双孢蘑菇(Agaricus bisporus)培养基质的链霉菌A06, 对双孢蘑菇产量提升31.5%[19]。
IAA是一类含有1个不饱和芳香族环和1个乙酸侧链的内源生长调节剂,对植物生长具有两重性作用。植物不同部位对其敏感度不同,一般根的敏感性大于芽,芽又大于茎。不同植物对IAA敏感度也不同。植物内生菌中产生大量生物活性物质,其中就包含IAA等吲哚衍生物[20]。最初报道链霉菌产生IAA的菌株是灰绿链霉菌(S. griseoviridis),经过制作成孢子悬浮液、菌体冻干粉末和上清液粉末制剂,施用后提高了黄瓜产量[21]。近期研究也表明链霉菌A1RT能分泌IAA等植物生长调节剂,促进了马铃薯块茎质量增加和茎部发育。IAA在链霉菌体内主要通过吲哚-3-乙酰胺(Trp-IAM-IAA)途径合成[22]。
土壤中存在氮、磷、钾等大量元素,同时也存在钙、铁、镁等微量元素。植物从土壤中吸收营养进行生长。固氮作用是指将大气中的氮转变为氨态氮或含氮化合物的过程,主要分为生物固氮与非生物固氮。而生物固氮主要通过一些微生物的固氮作用,将氮素提供给植物吸收。链霉菌能够协助一些微生物进行固氮作用,如链霉菌AUR4单独处理鹰嘴豆(Cicer arietinum),可以促进生长,提高种子质量和数量的同时也提升豆荚的质量与数量。链霉菌AUR4与链霉菌ARR2同时接种,对鹰嘴豆促生具有协同作用。链霉菌AUR4与链霉菌ARR2分别同固氮菌同时处理鹰嘴豆,提高了鹰嘴豆根部固氮瘤的产生与根内固氮酶活性[23]。灰黄链霉菌(S. griseo- flavus) P4能刺激大豆(Glycine max)根瘤的形成,促进生长。大豆品种‘Yezin-9’与‘Shan Seine’接种灰黄链霉菌P4后,根瘤形成分别提升75%与39%[24]。
嗜铁素是一类微生物产生的水溶性小分子化合物,能够特异性地结合铁离子[25]。按照结合铁的官能基团不同,嗜铁素可以分为4种类型:异羟肟酸型、儿茶酚型、羟基羧酸型和混合型[26]。微生物分泌嗜铁素,结合环境中的铁离子,一部分供给自身营养生长,一部分作为物质交换,传递给植物。在灭菌的土壤中,植物生长出现缺绿现象,表明微生物在植物营养吸收中占有重要地位[27]。分离的酸性疮痂链霉菌(S. acidiscabies)可以产生异羟肟酸型嗜铁素,能够促进镍离子胁迫下豇豆(Vigna ungui- culata)的生长[28]。接种分泌嗜铁素的链霉菌后,水稻种子的发芽率与生长势明显提高[29],橡胶树(Hevea brasiliensis)与葡萄(Vitis vinifera)也同样表现出良好的生长趋势[30]。盐屋链霉菌(S. sioyaensis)发酵上清液中富含嗜铁素,铬天青(CAS)平板法检测时,链霉菌菌落周围透明圈直径达到(11.75±0.76) mm,上清液处理橡胶树幼苗后,促进生长并提高株高,并对橡胶树根腐病起到防控的作用[25]。链霉菌RP1A- 12分泌嗜铁素,抑制S. rolfsii产生草酸,控制由S. rolfsii引起的花生茎腐病的蔓延,促进生长[31]。
链霉菌也可以提高植物对磷、钾等元素的吸收,促进生长。链霉菌能够提高鹰嘴豆植株体内生物碳和磷含量[32]。链霉菌提高植物体内矿质营养元素具有选择性,链霉菌可提高大麦体内氮、磷、钾含量,但钙含量并未见明显变化。千叶链霉菌(S. chibaensis)能提高蚕豆(Vicia faba)体内氮、磷、钾含量,并且促进营养蛋白的积累和提高矿质含量,提升产量[33]。
3 提升植物逆境耐受力植物在生长过程中,随着环境变化,会遭遇各种各样的逆境, 大体上可以分为生物逆境与非生物逆境。生物逆境主要为病虫与病害,可以通过外界喷施含有抑制病原菌或病害虫生长的药物和提高植物系统抗性来减轻病虫害。非生物逆境主要为干旱、盐碱、水渍与重金属污染等外在条件引起的不适合植物生长的环境因素,主要的预防措施是提高植物体内脯氨酸、抗氧化酶活性及相关抗性蛋白基因的表达来抵御(图 1)。
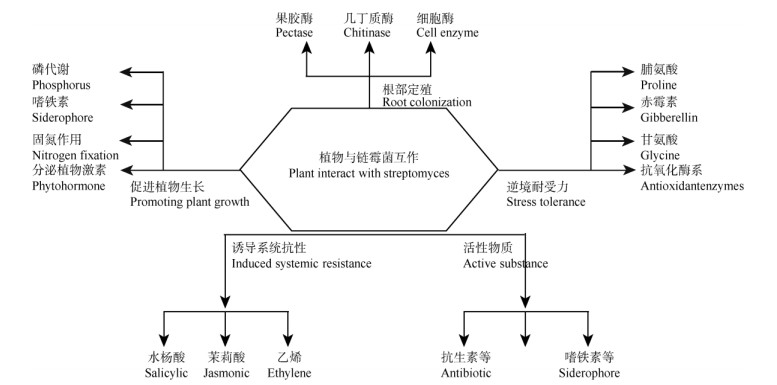
|
图 1 链霉菌与植物的互作(引自Vardharajula等[76], 略加修改) Fig. 1 Interaction between Streptomyces and plants (Revised from Vardharajula[76]) |
微生物在与植物互相作用的过程中逐渐形成了互惠互利的关系。植物供给微生物合适的生长环境,微生物提供植物必需的矿质营养。微生物协助植物抵抗逆境的方式有直接分泌具有抑菌抗虫作用的代谢物(化合物、几丁质酶、葡萄糖酶等)抑制病原菌生长,或者间接促进植物生长、提高系统抗性(抗氧化酶系、抗性蛋白)来抵御病原菌的入侵。
链霉菌分泌的代谢物包含多种类型的抑菌化合物,链霉菌的初期研究主要集中在医学抗生素的发掘上, 近期链霉菌分泌的代谢物在农业上也得到了广泛的应用。链霉菌代谢物中具有生物活性的物质主要分为生物碱类化合物、聚酮类化合物、萜类化合物、多肽类化合物、糖苷类化合物与酮类化合物[77],这些活性物质在农业生产与医学应用中发挥重要作用。白叶枯病菌造成水稻叶片枯萎,导致重大经济损失,其致病力与生物膜的形成能力有关, 抑制白叶枯病菌生物膜的形成,就能够减轻水稻白叶枯病害的发生和产量的降低。链霉菌菌株0320和4359可以抑制白叶枯病菌生物膜的形成,而不影响白叶枯病菌细胞的生长[78]。进一步研究表明, 是链霉菌分泌的化合物邻氨基苯甲酰氨在起作用, 由于并未影响白叶枯病菌细胞的生长,所以未来出现对该化合物有耐受性的白叶枯病菌的几率较小[78]。链霉菌代谢物不仅自身具有抑菌活性,也可以活化其他无抑菌活性的物质。最近的研究表明,链霉菌代谢物还能够合成纳米粒子,进而增强代谢物抑制病原菌生长的能力。Vijayabharathi等[79]的研究表明链霉菌SAI-25具有合成AgNPs的能力,通过在上清液中加入AgNO3, 可以反应生成AgNPs, AgNPs可以有效抑制芽腐病菌(Macrophomina phaseolina)的生长,降低高粱(Sorghum bicolor)芽腐病的发生。
在链霉菌与病原真菌的寄生过程中,几丁质酶是一种关键因素。微生物通过分泌几丁质酶降解病原真菌细胞壁,从而进入病原真菌体内并获取营养。这也为植物病害防治提供了有效措施, 盐屋链霉菌能够分泌几丁质酶抑制病菌生长,降低橡胶树(Hevea brasiliensis)白根病的发病率。Shivalee等[80]通过添加胶体几丁质和优化培养条件等措施,使得链霉菌KLSL55产生的几丁质酶含量提升14.30倍。Gao等[81]从链霉菌ATCC 27414中克隆到一种新的几丁质酶基因,转入大肠杆菌中得到表达,且几丁质酶活性具有pH与温度稳定性。
链霉菌作用于植物根部,可以激活叶片对于病原菌的抵御能力,诱导植物产生系统性抗性。植物系统性抗性主要通过水杨酸等信号途径进行传导, 诱导抗氧化酶系统、脯氨酸和蛋白酶的改变,增强对病虫病害的防御,促进植物生长。Sunpapao等[82]的研究表明,链霉菌V76-12对米弯孢菌(Curvularia oryzae)生长的抑制率达到85.71%,椰子(Cocos nuci- fera)幼苗经链霉菌V76-12处理后,病情指数由75.33%降至21%,提高了幼苗的抗氧化酶系统(PAL、PPO和POD)的活性。Shariffah-Muzaimah等[83]筛选的链霉菌AGA347,使椰子幼苗茎腐病病情指数降低73.1%。链霉菌JD211可以诱导水稻的系统抗性,几丁质酶与抗病相关蛋白1 (PR1)的基因表达量提升, 抗氧化酶(PAL与葡聚糖酶)的活性得到增强, 有效控制水稻稻瘟病的蔓延[84]。Streptomyces rochei SM3通过提升鹰嘴豆的抗氧化酶(POD、CAT和PPO等)活性和酚类物质含量,增强抵御葡萄球菌的侵染能力[35]。
链霉菌不仅能够协助植物抵御病虫病害的侵染,也能够帮助植物顺利渡过非生物逆境的不利影响,并改善生态环境。温室与大田试验表明,链霉菌C2012和C801可以提高薄荷地上部分鲜质量和干质量[12],薄荷在水渍逆境中,鲜质量和干质量都降低,但接种链霉菌后有效缓解了水渍逆境带来的负面影响,有效提高鲜质量和干质量,提高了薄荷油与挥发油含量。链霉菌MM10也能够显著提高小麦对高盐土壤的耐受力,并且提高产量[52]。二氯喹啉酸是一种广泛应用于水稻等作物中的除草剂,可有效控制杂草生长,由于其较难降解,长期大量使用后,在土壤中残留过多,对环境造成不良影响。Lang等[85]报道链霉菌AB-H对二氯喹啉酸具有降解作用,实验室研究表明,其降解率达到97.2%, 应用于土壤后, 降解率为87.5%。因此,链霉菌AB-H在生态环境的优化方面具有良好的市场前景。链霉菌HBUM174787具有产生生物絮凝剂的能力,絮凝剂具有羟基、羧基、甲氧基与氨基基团,能够降低63.1%的河流含氧量和46.6%的肉制品加工废水含氧量, 使其混浊度分别降低84.3%和75.6%,在河流污染治理方面具有广阔的应用前景[86]。龟裂链霉菌能够从水溶液中吸收重金属(铅和镍),为治理重金属污染的土壤提供思路[87]。
4 链霉菌在农业生产中的应用目前,国内生物菌肥产业还处于初步发展阶段, 微生物菌肥生产厂家大约在1 000家左右,微生物肥料年产量在1.0×108 t水平,推广应用面积1.3× 107 hm2[88], 产品数量、类型与企业分布在逐渐走向多元化的过程中。随着人们对于生态环境的关注, 生物菌肥的需求将逐步递增。
微生物在农业生产上的应用形态主要有粉剂、颗粒剂和水剂。粉剂和颗粒剂便于运输,水剂的活性更好。具体应用何种形态的微生物制剂,需随菌种不同选择更加高效的成品制剂。微生物制剂的主要问题是活性问题,大部分微生物受环境影响较大,温度变化会对微生物制剂活性产生较大影响。关于微生物制剂的保存条件,湿度与温度都是需要考虑的因素。在应用上,主要处理方式有浸种、灌根与喷施叶片,浸种方法省时省力,是目前微生物处理的主要方式;灌根需要微生物菌量大,但效果持久;喷施叶片具有见效快的优点。近期有一种含金黄垂直链霉菌HN6的可湿性粉剂,是将润湿剂、分散剂、稳定剂、填料投入到气流粉碎机中粉碎, 过325目筛,降温后,加入金黄垂直链霉菌HN6液体发酵物,继续粉碎,使其最终水分含量≤5%, 活性孢子数为(0.1~10)×109 g-1,该药剂属于微生物活体制剂,对香蕉枯萎病有显著防治效果,对其它病害也有较好的防治作用,同时对生态环境无毒副作用,保障了农产品的质量与安全[89]。货架期短是生物菌肥应用过程中,需要克服的一个技术难点, 开发出高效、贮存期长的生物菌肥是农业生产上所急需的。目前的研究大多数以实验室以及温室等控制条件为主,在这种条件下,链霉菌作为生防菌能取得良好的效果。但在大田等复杂环境中,链霉菌应用的成功报道还相对较少,今后需要进一步在大田等复杂环境中进行链霉菌生物防治的试验。
| [1] |
Actinomycetes Classification Group of Institute of Microbiology in Chinese Academy of Sciences. Identification Manual for Streptomyces[M]. Beijing: Science Press, 1975: 6-12. 中国科学院微生物研究所放线菌分类组. 链霉菌鉴定手册[M]. 北京: 科学出版社, 1975: 6-12. |
| [2] |
BUSH M J, TSCHOWRI N, SCHLIMPERT S, et al. c-di-GMP signalling and the regulation of developmental transitions in streptomycetes[J]. Nat Rev Microbiol, 2015, 13(12): 749-760. DOI:10.1038/nrmicro3546 |
| [3] |
KIM Y C, LEVEAU J, McCSPADDEN G B B, et al. The multifactorial basis for plant health promotion by plant-associated bacteria[J]. Appl Environ Microb, 2011, 77(5): 1548-1555. DOI:10.1128/AEM.01867-10 |
| [4] |
JáNOS B. Bioactive microbial metabolites[J]. J Antibiot, 2005, 58(1): 1-26. DOI:10.1038/ja.2005.1 |
| [5] |
LIU X Y, MA Y C. Green fluorescent protein marker of biocontrol Streptomyces SSD49 and its colonization on the Populus tomentosa somaclone[J]. Biotechnol Bull, 2016, 32(9): 197-202. 刘晓瑜, 马玉超. 生防链霉菌SSD49的绿色荧光蛋白标记及其在毛白杨组培苗中的定殖[J]. 生物技术通报, 2016, 32(9): 197-202. DOI:10.13560/j.cnki.biotech.bull.1985.2016.09.026 |
| [6] |
DAI P B, LAN X J, ZHANG W W, et al. Identification, colonization and disease suppressive effect of strain SC11 against cotton Fusarium wilt[J]. Acta Phytopath Sin, 2016, 46(2): 273-279. 戴蓬博, 蓝星杰, 张伟卫, 等. 生防菌株SC11的鉴定、定殖及对棉花枯萎病防治效果研究[J]. 植物病理学报, 2016, 46(2): 273-279. DOI:10.13926/j.cnki.apps.2016.02.016 |
| [7] |
QIAO T M, ZHENG L, ZHANG J, et al. Dynamic detection for coloni- zation of Streptomyces purpeofuscus in Juglans sigllata rhizosphere[J]. J Nanjing For Univ (Nat Sci), 2015, 39(5): 21-26. 谯天敏, 郑磊, 张静, 等. 绛红褐链霉菌的根际定殖能力动态监测[J]. 南京林业大学学报(自然科学版), 2015, 39(5): 21-26. DOI:10.3969/j.issn.1000-2006.2015.05.004 |
| [8] |
SHIRLING E B, GOTTLIEB D. Methods for characterization of Streptomyces species[J]. Int J Syst Bacteriol, 1966, 16(3): 313-340. DOI:10.1099/00207713-16-3-313 |
| [9] |
LECHEVALIER M P, LECHEVALIER H. Chemical composition as a criterion in the classification of aerobic actinomycetes[J]. Int J Syst Bacteriol, 1970, 20(4): 435-443. DOI:10.1099/00207713-20-4-435 |
| [10] |
WOESE C R, KANDLER O, WHEELIS M L. Towards a natural system of organisms: Proposal for the domains archaea, bacteria, and eucarya[J]. Proc Natl Acad Sci USA, 1990, 87(12): 4576-4579. DOI:10.1073/pnas.87.12.4576 |
| [11] |
XIA Q H. Effects of an endophytic Streptomyces sp. on growth and artemisinin biosynthesis of Artemisia annua L.[D]. Suzhou: Soochow University, 2016: 21-26. 夏倩华.内生链霉菌(Streptomyces sp.)对黄花蒿生长和青蒿素合成的影响[D].苏州: 苏州大学, 2016: 21-26. http://cdmd.cnki.com.cn/Article/CDMD-10285-1016226008.htm |
| [12] |
SUN P Y. Study on the isolation, identification and antimicrobial activity of Endophytic actinomycetes against cucumber fusarium wilt[D]. Harbin: Northeast Agricultural University, 2017: 23-38. 孙鹏宇.黄瓜枯萎病拮抗内生放线菌分离、鉴定与抑菌活性研究[D].哈尔滨: 东北农业大学, 2017: 23-38. http://cdmd.cnki.com.cn/Article/CDMD-10224-1017150205.htm |
| [13] |
NAHAR K, GOYER C, ZEBARTH B J, et al. Pathogenic Streptomyces spp. abundance affected by potato cultivars[J]. Phytopathology, 2018, 108(9): 1046-1055. DOI:10.1094/PHYTO-03-18-0075-R |
| [14] |
KEILHOFER N, NACHTIGALL J, KULIK A, et al. Streptomyces AcH 505 triggers production of a salicylic acid analogue in the fungal pathogen Heterobasidion abietinum that enhances infection of Norway spruce seedlings[J]. Anton Leeuw, 2018, 111(5): 691-704. DOI:10.1007/s10482-018-1017-9 |
| [15] |
WANG Z, PAND F, GU C C, et al. Establishment and optimization of Streptomyces chartreusi WZS021 transconjugation system[J]. J S Agric, 2017, 48(4): 581-586. 王震, 庞妃, 顾彩彩, 等. 固氮链霉菌Streptomyces chartreusi WZS021接合转移系统的建立及优化[J]. 南方农业学报, 2017, 48(4): 581-586. DOI:10.3969/j.issn.2095-1191.2017.04.003 |
| [16] |
WU Q, NI M, LIU W C, et al. Omics for understanding the mecha- nisms of Streptomyces lydicus A01 promoting the growth of tomato seedlings[J]. Plant Soil, 2018, 431(1/2): 129-141. DOI:10.1007/s11104-018-3750-2 |
| [17] |
JACOB S, SAJJALAGUDDAM R R, SUDINI H K. Streptomyces sp. RP1A-12 mediated control of peanut stem rot caused by Sclerotium rolfsii[J]. J Integr Agric, 2018, 17(4): 892-900. DOI:10.1016/S2095-3119(17)61816-1 |
| [18] |
ESMAEIL Z N S, SADEGHI A, MORADI P. Streptomyces strains alleviate water stress and increase peppermint (Mentha piperita) yield and essential oils[J]. Plant Soil, 2019, 434(1/2): 441-452. DOI:10.1007/s11104-018-3862-8 |
| [19] |
ŠANTRIĆ L, POTOČNIK I, RADIVOJEVIĆ L, et al. Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus bisporus[J]. J Environ Sci Heal B, 2018, 53(10): 677-684. DOI:10.1080/03601234.2018.1474559 |
| [20] |
LIN L, XU X D. Indole-3-acetic acid production by endophytic Strep- tomyces sp. En-1 isolated from medicinal plants[J]. Curr Microbiol, 2013, 67(2): 209-217. DOI:10.1007/s00284-013-0348-z |
| [21] |
TUOMI T, LAAKSO S, ROSENQVIST H. Indole-3-acetic acid (IAA) production by a biofungicide Streptomyces griseoviridis strain[J]. Ann Bot Fenn, 1994, 31(1): 59-63. |
| [22] |
MANULIS S, SHAFRIR H, EPSTEIN E, et al. Biosynthesis of indole- 3-acetic acid via the indole-3-acetamide pathway in Streptomyces spp.[J]. Microbiology, 1994, 140(5): 1045-1050. DOI:10.1099/13500872-140-5-1045 |
| [23] |
VIJAYABHARATHI R, GOPALAKRISHNAN S, SATHYA A, et al. Deciphering the tri-dimensional effect of endophytic Streptomyces sp. on chickpea for plant growth promotion, helper effect with Mesorhi- zobium ciceri and host-plant resistance induction against Botrytis cinerea[J]. Microb Pathog, 2018, 122: 98-107. DOI:10.1016/j.micpath.2018.06.019 |
| [24] |
HTWE A Z, YAMAKAWA T. Low-density co-inoculation with Bradyr- hizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 promotes plant growth and nitrogen fixation in soybean cultivars[J]. Amer J Plant Sci, 2016, 7(12): 1652-1661. DOI:10.4236/ajps.2016.712156 |
| [25] |
GUERINOT M L. Microbial iron transport[J]. Annu Rev Microbiol, 1994, 48(1): 743-772. DOI:10.1146/annurev.mi.48.100194.003523 |
| [26] |
BOUKHALFA H, CRUMBLISS A L. Chemical aspects of siderophore mediated iron transport[J]. Biometals, 2002, 15(4): 325-339. DOI:10.1023/a:1020218608266 |
| [27] |
MASALHA J, KOSEGARTEN H, ELMACI Ö, et al. The central role of microbial activity for iron acquisition in maize and sunflower[J]. Biol Fert Soils, 2000, 30(5/6): 433-439. DOI:10.1007/s003740050021 |
| [28] |
DIMKPA C, SVATOŠ A, MERTEN D, et al. Hydroxamate sidero- phores produced by Streptomyces acidiscabies E13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress[J]. Can J Microbiol, 2008, 54(3): 163-172. DOI:10.1139/w07-130 |
| [29] |
TAMREIHAO K, NIMAICHAND S, CHANU S B, et al. Acidotolerant Streptomyces sp. MBRL 10 from limestone quarry site showing anta- gonism against fungal pathogens and growth promotion in rice plants[J]. J King Saud Univ Sci, 2018, 30(2): 143-152. DOI:10.1016/j.jksus.2016.10.003 |
| [30] |
NAKAEW N, RANGJAROEN C, SUNGTHONG R. Utilization of rhizospheric Streptomyces for biological control of Rigidoporus sp. causing white root disease in rubber tree[J]. Eur J Plant Pathol, 2015, 142(1): 93-105. DOI:10.1007/s10658-015-0592-0 |
| [31] |
JACOB S, SAJJALAGUDDAM R R, KUMAR K V K, et al. Assessing the prospects of Streptomyces sp. RP1A-12 in managing groundnut stem rot disease caused by Sclerotium rolfsii Sacc[J]. J Gen Plant Pathol, 2016, 82(2): 96-104. DOI:10.1007/s10327-016-0644-0 |
| [32] |
GOPALAKRISHNAN S, SRINIVAS V, ALEKHYA G, et al. Evaluation of broad-spectrum Streptomyces sp. for plant growth promotion traits in chickpea (Cicer arietinum L.)[J]. Phil Agric Sci, 2015, 98(3): 270-278. |
| [33] |
HEWEDY M A. Associative effect of the rhizobacteria Streptomyces chibaensis and commercial biofertilizers on the growth, yield and nutritional value of Vicia faba[J]. Egypt J Biol Pest Co, 2011, 21(2): 219-225. |
| [34] |
de KLERK A, MCLEOD A, FAURIE R, et al. Net blotch and necrotic warts caused by Streptomyces scabies on pods of peanut (Arachis hypogaea)[J]. Plant Dis, 2007, 81(8): 958. DOI:10.1094/PDIS.1997.81.8.958B |
| [35] |
SRIVASTAVA S, PATEL J S, SINGH H B, et al. Streptomyces rochei SM3 induces stress tolerance in chickpea against Sclerotinia sclera- tiorum and NaCl[J]. J Phytopathol, 2015, 163(7/8): 583-592. DOI:10.1111/jph.12358 |
| [36] |
ZHOU D B, JING T, QI D F, et al. Isolation and identification of Strep- tomyces lunalinharesii and its control effect on the banana fusarium wilt disease[J]. Acta Hort Sin, 2017, 44(4): 664-674. 周登博, 井涛, 起登凤, 等. 抗香蕉枯萎病菌的卢娜林瑞链霉菌的分离及防效鉴定[J]. 园艺学报, 2017, 44(4): 664-674. DOI:10.16420/j.issn.0513-353x.2016-0598 |
| [37] |
MA J N, LIU Y T, LI Y L, et al. Effects and mechanism of two Strep- tomyces strains on promoting plant growth and increasing grain yield of maize[J]. Chin J Appl Ecol, 2017, 28(1): 315-326. 马军妮, 刘玉涛, 李玉龙, 等. 两株链霉菌对玉米的促生增产作用及机理[J]. 应用生态学报, 2017, 28(1): 315-326. DOI:10.13287/j.1001-9332.201701.038 |
| [38] |
POSTOLAKY O, BALTSAT K, BURTSEVA S, et al. Effect of Strepto- myces metabolites on some physiological parameters of maize seeds[J]. Bull Univ Agric Sci Vet, 2012, 69(1): 23-29. |
| [39] |
BRESSAN W, FIGUEIREDO J E F. Biological control of Stenocar- pella maydis in maize seed with antagonistic Streptomyces sp. isolates[J]. J Phytopathol, 2005, 153(10): 623-626. DOI:10.1111/j.1439-0434.2005.01014.x |
| [40] |
BRESSAN W, FIGUEIREDO J E F. Efficacy and dose-response relationship in biocontrol of Fusarium disease in maize by Strepto- myces spp.[J]. Eur J Plant Pathol, 2008, 120(3): 311-316. DOI:10.1007/s10658-007-9220-y |
| [41] |
QI B S, YANG W X, LIU D Q. A preliminary study on antagonism of Streptomyces spp. against Curvularia leaf spot of maize[J]. J Agric Univ Hebei, 2000, 23(3): 76-79. 祁碧菽, 杨文香, 刘大群. 链霉菌对玉米弯孢霉菌抑制作用的初步研究[J]. 河北农业大学学报, 2000, 23(3): 76-79. DOI:10.3969/j.issn.1000-1573.2000.03.018 |
| [42] |
NA J, HUI X, LI W J, et al. Field evaluation of Streptomyces rubro- griseus HDZ-9-47 for biocontrol of Meloidogyne incognita on tomato[J]. J Integr Agric, 2017, 16(6): 1347-1357. DOI:10.1016/S2095-3119(16)61553-8 |
| [43] |
SABARATNAM S, TRAQUAIR J A. Mechanism of antagonism by Streptomyces griseocarneus (strain Di944) against fungal pathogens of greenhouse-grown tomato transplants[J]. Can J Plant Pathol, 2015, 37(2): 197-211. DOI:10.1080/07060661.2015.1039062 |
| [44] |
LI Q L, NING P, ZHENG L, et al. Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit[J]. Biol Control, 2012, 61(2): 113-120. DOI:10.1016/j.iocontrol.2011.10.014 |
| [45] |
JAYAKUMAR J. Streptomyces avermitilis as a biopesticide for the management of root knot nematode Meloidogyne incognita in tomato[J]. Karnataka J Agric Sci, 2009, 22(3): 564-566. |
| [46] |
SABARATNAM S, TRAQUAIR J A. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplants[J]. Biol Control, 2002, 23(3): 245-253. DOI:10.1006/bcon.2001.1014 |
| [47] |
LIU P P. The ultraviolet mutagenesis of antagonistic Streptomyces sp. CC5 and biocontrol potential against potato scab[D]. Nanjing: Nanjing Agricultural University, 2016: 40-42. 刘萍萍.生防链霉菌Streptomyces sp. CC5的紫外诱变育种及其在马铃薯疮痂病防控中的应用[D].南京: 南京农业大学, 2016: 40-42. http://cdmd.cnki.com.cn/Article/CDMD-10307-1017261907.htm |
| [48] |
ZHANG J, WANG L M, LI Y H, et al. Biocontrol of cereal cyst nematode by Streptomyces anulatus isolate S07 [J]. Australas Plant Pathol, 2016, 45(1): 57-64. DOI:10.1007/s13313-015-0385-0 |
| [49] |
TOUMATIA O, COMPANT S, YEKKOUR A, et al. Biocontrol and plant growth promoting properties of Streptomyces mutabilis strain IA1 isolated from a Saharan soil on wheat seedlings and visualization of its niches of colonization[J]. S Afr J Bot, 2016, 105: 234-239. DOI:10.1016/j.sajb.2016.03.020 |
| [50] |
EL-SHANSHOURY A R. Growth promotion of wheat seedlings by Streptomyces atroolivaceus[J]. J Agron Crop Sci, 1989, 163(2): 109-114. DOI:10.1111/j.1439-037X.1989.tb00743.x |
| [51] |
TAHVONEN R, HANNUKKALA A, AVIKAINEN H. Effect of seed dressing treatment of Streptomyces griseoviridis on barley and spring wheat in field experiments[J]. Agric Food Sci, 1995, 4(4): 419-427. DOI:10.23986/afsci.72619 |
| [52] |
ALY M M, EL SAYED H E S A, JASTANIAH S D. Synergistic effect between Azotobacter vinelandii and Streptomyces sp. isolated from saline soil on seed germination and growth of wheat plant[J]. J Amer Sci, 2012, 8(5): 667-676. |
| [53] |
MANHAS R K, KAUR T. Biocontrol potential of Streptomyces hydrogenans strain DH16 toward Alternaria brassicicola to control damping off and black leaf spot of Raphanus sativus[J]. Front Plant Sci, 2016, 7: 1869. DOI:10.3389/fpls.2016.01869 |
| [54] |
GAO X N, HE Q R, JIANG Y, et al. Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae Xjy and its biocontrol efficacies against Fulvia fulva and Botryosphaeria dothidea[J]. J Phytopathol, 2016, 164(3): 155-165. DOI:10.1111/jph.12440 |
| [55] |
PENG J, WU X P, ZHANG K S, et al. Separation of active compounds against Pyricularia oryzae from sponge-associated actinobacteria Streptomyces sp. A01059[J]. Chin Agric Sci Bull, 2009, 25(9): 51-54. 彭杰, 吴晓鹏, 张开山, 等. 海绵共附生放线菌Streptomyces sp. A01059抗稻瘟病活性物质的分离研究[J]. 中国农学通报, 2009, 25(9): 51-54. |
| [56] |
ZARANDI M E, BONJAR G H S, DEHKAEI F P, et al. Biological control of rice blast (Magnaporthe oryzae) by use of Streptomyces sindeneusis isolate 263 in greenhouse[J]. Amer J Appl Sci, 2009, 6(1): 194-199. DOI:10.3844/ajassp.2009.194.199 |
| [57] |
HUANG S W, YU L Q, WASTON A K. Inhibiting efficacy of metabolites of Streptomyces lavendulohygtroscopicus and its ultraviolet induced strain on two rice diseases[J]. Chin Rice Res Newslett, 2000, 8(2): 5-6. |
| [58] |
XUE L, GU M Y, XU W L, et al. Antagonistic Streptomyces enhances defense-related responses in cotton for biocontrol of wilt caused by phytotoxin of Verticillium dahliae[J]. Phytoparasitica, 2016, 44(2): 225-237. DOI:10.1007/s12600-016-0517-2 |
| [59] |
XIAO K, KINKEL L L, SAMAC D A. Biological control of Phyto- phthora root rots on alfalfa and soybean with Streptomyces[J]. Biol Control, 2002, 23(3): 285-295. DOI:10.1006/bcon.2001.1015 |
| [60] |
SHEN T, ZHANG Y Y, WANG C, et al. Study on solid fermentation of Streptomyces albospinus CT205 and biocontrol effect against straw- berry root rot[J]. J Nanjing Agric Univ, 2015, 38(4): 596-601. 沈婷, 张园园, 王辰, 等. 白刺链霉菌(Streptomyces albospinus) CT205菌株固体发酵及防控草莓根腐病的研究[J]. 南京农业大学学报, 2015, 38(4): 596-601. DOI:10.7685/j.issn.1000-2030.2015.04.011 |
| [61] |
CHENG G L. Study on mutation breeding of Streptomyces felleus and the biocontrol of Sclerotinia sclerotiorum by mutant strains[D]. Chengdu: Sichuan Agricultural University, 2014: 47-55. 程光龙.苦胆链霉菌Streptomyces felleus的诱变选育及其对油菜菌核病的生物防治[D].成都: 四川农业大学, 2014: 47-55. http://cdmd.cnki.com.cn/Article/CDMD-10626-1016048608.htm |
| [62] |
HAN X Y, ZHANG C S, CHEN X, et al. Screening, identification and biocontrol effect of antagonistic Streptomyces strain Tra69 against tobacco bacterial wilt[J]. Plant Dis Pest, 2012, 3(1): 10-13, 32. |
| [63] |
BOUKAEW S, CHUENCHIT S, PETCHARAT V. Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili pepper[J]. Biocontrol, 2011, 56(3): 365-374. DOI:10.1007/s10526-010-9336-4 |
| [64] |
EZZIYYANI M, REQUENA M E, EGEA-GILABERT C, et al. Biolo- gical control of Phytophthora root rot of pepper using Trichoderma harzianum and Streptomyces rochei in combination[J]. J Phytopathol, 2007, 155(6): 342-349. DOI:10.1111/j.1439-0434.2007.01237.x |
| [65] |
SINGH A K, CHHATPAR H S. Combined use of Streptomyces sp. A6 and chemical fungicides against fusarium wilt of Cajanus cajan may reduce the dosage of fungicides required in the field[J]. Crop Prot, 2011, 30(7): 770-775. DOI:10.1016/j.cropro.2011.03.015 |
| [66] |
MOHAMED B, BENALI S. The talc formulation of Streptomyces antagonist against Mycosphaerella foot rot in pea (Pisum sativum L.) seedlings[J]. Arch Phytopathol Plant Prot, 2010, 43(5): 438-445. DOI:10.1080/03235400701851027 |
| [67] |
MANSOUR M T M, MOHAMED S H, ZAYED S M E, et al. Field evaluation of some Streptomyces isolates to suppress powdery mildew of flax (Sakha cultivar)[J]. Pak J Biotechnol, 2010, 7(1/2): 101-107. |
| [68] |
YOUSSEF Y A, EL-TARABILY K A, HUSSEIN A M. Plectosporium tabacinum root rot disease of white lupine (Lupinus termis Forsk.) and its biological control by Streptomyces species[J]. J Phytopathol, 2001, 149(1): 29-33. DOI:10.1046/j.1439-0434.2001.00565.x |
| [69] |
SAMAC D A, KINKEL L L. Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp.[J]. Plant Soil, 2001, 235(1): 35-44. DOI:10.1023/a:1011820002779 |
| [70] |
CHEAH L H, KENT G, GOWERS S. Brassica crops and a Strepto- myces sp. as potential biocontrol for clubroot of brassicas[C]// ZYDENBOS S M. New Zealand Plant Protection. New Zealand: New Zealand Plant Protection Society, 2001: 80-83.
|
| [71] |
CHEAH L H, VEERAKONE S, KENT G. Biological control of club- root on cauliflower with Trichoderma and Streptomyces spp.[C]// ZYDENBOS S M. New Zealand Plant Protection. New Zealand: New Zealand Plant Protection Society, 2000: 18-21.
|
| [72] |
HILTUNEN L H, LINFIELD C A, WHITE J G. The potential for the biological control of basal rot of Narcissus by Streptomyces sp.[J]. Crop Prot, 1995, 14(7): 539-542. DOI:10.1016/0261-2194(95)00068-2 |
| [73] |
DIXIT R B, GUPTA J S. Studies on the biological control of leaf blotch disease of barley by Streptomyces olivaceus[J]. Acta Bot Ind, 1980, 8(2): 190-192. |
| [74] |
KUSAKARI S, OKADA K, KAWARATANI M, et al. Suppression of Verticillium wilt of egg plants by Streptomyces sp. (C-26) in infested soil[J]. P Kan Plant Prot Soc, 1990, 32(1): 17-20. DOI:10.4165/kapps1958.32.0_17 |
| [75] |
O'BRIEN J G, BLANCHETTE R A, SUTHERLAND J B. Assessment of Streptomyces spp. from elms for biological control of Dutch elm disease[J]. Plant Dis, 1984, 68(2): 104-106. DOI:10.1094/PD-69-104 |
| [76] |
VARDHARAJULA S, SKZ A, VURUKONDA S S K P, et al. Plant growth promoting endophytes and their interaction with plants to alle- viate abiotic stress[J]. Curr Biotechnol, 2017, 6(3): 252-263. DOI:10.2174/2211550106666161226154619 |
| [77] |
CHENG J, ZHANG X L, ZHAO J Y, et al. The recent progress of study on secondary metabolites of Streptomyces[J]. Chin J Antibiot, 2015, 40(10): 791-800. 程举, 张孝龙, 赵江源, 等. 近年链霉菌次生代谢产物研究进展[J]. 中国抗生素杂志, 2015, 40(10): 791-800. DOI:10.3969/j.issn.1001-8689.2015.10.015 |
| [78] |
HAM Y, KIM T J. Anthranilamide from Streptomyces spp. inhibited Xanthomonas oryzae biofilm formation without affecting cell growth[J]. Appl Biol Chem, 2018, 61(6): 673-680. DOI:10.1007/s13765-018-0405-1 |
| [79] |
VIJAYABHARATHI R, SATHYA A, GOPALAKRISHNAN S. Extra- cellular biosynthesis of silver nanoparticles using Streptomyces griseo- planus SAI-25 and its antifungal activity against Macrophomina phaseolina, the charcoal rot pathogen of sorghum[J]. Biocatal Agric Biotechnol, 2018, 14: 166-171. DOI:10.1016/j.bcab.2018.03.006 |
| [80] |
SHIVALEE A, LINGAPPA K, MAHESH D. Influence of bioprocess variables on the production of extracellular chitinase under submerged fermentation by Streptomyces pratensis strain KLSL55 [J]. J Genet Eng Biotechnol, 2018, 16(2): 421-426. DOI:10.1016/j.jgeb.2017.12.006 |
| [81] |
GAO L, SUN J N, SECUNDO F, et al. Cloning, characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414[J]. Food Chem, 2018, 261: 329-336. DOI:10.1016/j.foodchem.2018.04.068 |
| [82] |
SUNPAPAO A, CHAIRIN T, ITO S I. The biocontrol by Streptomyces and Trichoderma of leaf spot disease caused by Curvularia oryzae in oil palm seedlings[J]. Biol Control, 2018, 123: 36-42. DOI:10.1016/j.biocontrol.2018.04.017 |
| [83] |
SHARIFFAH-MUZAIMAH S A, IDRIS A S, MADIHAH A Z, et al. Characterization of Streptomyces spp. isolated from the rhizosphere of oil palm and evaluation of their ability to suppress basal stem rot disease in oil palm seedlings when applied as powder formulations in a glass- house trial[J]. World J Microb Biot, 2018, 34(1): 15. DOI:10.1007/s11274-017-2396-1 |
| [84] |
SHAO Z Y, LI Z, FU Y H, et al. Induction of defense responses against Magnaporthe oryzae in rice seedling by a new potential biocontrol agent Streptomyces JD211[J]. J Bas Microb, 2018, 58(8): 686-697. DOI:10.1002/jobm.201800100 |
| [85] |
LANG Z, QI D, DONG J J, et al. Isolation and characterization of a quinclorac-degrading Actinobacteria Streptomyces sp. strain AH-B and its implication on microecology in contaminated soil[J]. Chemosphere, 2018, 199: 210-217. DOI:10.1016/j.chemosphere.2018.01.133 |
| [86] |
AGUNBIADE M, POHL C, ASHAFA O. Bioflocculant production from Streptomyces platensis and its potential for river and waste water treatment[J]. Braz J Microbiol, 2018, 49(4): 731-741. DOI:10.1016/j.bjm.2017.02.013 |
| [87] |
YOUS R, MOHELLEBI F, CHERIFI H, et al. Competitive biosorption of heavy metals from aqueous solutions onto Streptomyces rimosus[J]. Korean J Chem Eng, 2018, 35(4): 890-899. DOI:10.1007/s11814-018-0004-1 |
| [88] |
MA C B, SHI M Y. Development of microbial fertilizer industry in China[J]. China Agric Technol Ext, 2016, 32(2): 13-18. 马常宝, 史梦雅. 我国微生物肥料产业发展状况[J]. 中国农技推广, 2016, 32(2): 13-18. DOI:10.3969/j.issn.1002-381X.2016.02.004 |
| [89] |
WANG L Y, WANG Q, LUO Y P. Disease preventing and growth promoting effects of Streptomyces aureoverticillatus strain HN6 on banana[J]. J NW Agric For Univ (Nat Sci), 2015, 43(5): 163-167. 王兰英, 王琴, 骆焱平. 金黄垂直链霉菌HN6对香蕉的防病促生作用[J]. 西北农林科技大学学报(自然科学版), 2015, 43(5): 163-167. DOI:10.13207/j.cnki.jnwafu.2015.05.008 |
 2019, Vol. 27
2019, Vol. 27



