2. 中国热带农业科学院热带生物技术研究所, 海南省黎药资源天然产物研究与利用重点实验室, 海口 571101
2. Hainan Key Laboratory for Research and Development of Natural Products from Li Folk Medicine, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou 571101, China
血竭(dragon's blood)是一种传统的名贵中药, 具有活血化瘀、收敛止血、消肿止痛、补血补虚的功效,用于治疗外伤出血、跌打损伤、消肿止痛、瘀滞作痛等各类血症,自古以来广泛应用于世界很多国家。国内使用的血竭有进口与国产之分,进口血竭主要是棕榈科(Palmacea)黄藤属(Daemonorps)植物果实的树脂,国产血竭是由龙血树属(Dracaena)植物在受到伤害(物理、化学或生物等因素)后在茎干中分泌的红色树脂[1-2],其基源植物包括剑叶龙血树(D. cochinchinensis)和海南龙血树(D. cambo- diana)[3]。基于血竭显著的疗效,近年来市场需求迅速增长,但是自然界中血竭的形成极其缓慢,需要几年甚至几十年,仅依靠采集野生血竭不能满足市场需求,且国产血竭基源植物剑叶龙血树和海南龙血树均为国家二级濒危保护植物[4],植物资源相当有限。因此,采用人工诱导的方法,加速血竭的形成,有助于医药用血竭的可持续利用。但是,目前关于人工诱导血竭的化学成分和生物活性研究较少,主要由本课题组前期对人工诱导的海南龙血树所产血竭的化学成分和生物活性进行了研究[5-7]。本研究采用硅胶柱色谱、反相硅胶柱色谱、Sephadex LH-20凝胶柱色谱以及HPLC等手段对人工诱导血竭继续进行分离纯化及其结构鉴定,丰富人工诱导血竭化学成分的研究基础,为人工诱导血竭代替野生血竭提供理论依据。
1 材料和方法 1.1 材料人工诱导血竭是采用输液法将本课题组自主创新研制的诱导剂ITBB001输入到2年生海南龙血树的枝条中,诱导1年后,于2014年11月采集树干,将其中变红的部分刮下,晒干,即得到人工诱导血竭(600.0 g)。经中国热带农业科学院热带生物技术研究所王军副研究员鉴定其基源植物为海南龙血树(Dracaena cambodiana),凭证标本(BL201411)存放于中国热带农业科学院热带生物技术研究所。
1.2 仪器和试剂Bruker AV-500型超导核磁共振仪(TMS为内标); Autospec-3000质谱仪;Agilent Technologies 1260高分析型校液相色谱仪(美国安捷伦公司); Labal- liance PC-4000半制备型高效液相色谱仪(美国兰博公司);Laborta 4001 (2 L)旋转蒸发仪(德国海道尔夫公司);Rotavac Valve Tec二级隔膜泵(德国海道尔夫公司);CA-1111冷却水循环装置(东京理化器械株式会社);BP221S万分之一电子秤(北京赛多利斯天平有限公司);超净工作台(上海博讯实业有限公司医疗设备厂);电热鼓风干燥箱(上海一恒科技有限公司);超纯水装置(厦门锐思捷水纯化技术有限公司)。柱层析硅胶(200~300目,60~80目)和薄层层析硅胶板(青岛海洋化工厂),Sephadex LH-20、RP- 18 (Fuji公司),常用分析纯有机溶剂(广州化工和天津大茂等);AR级浓硫酸(博滨岭化工有限公司); 氘代试剂(Merck公司);色谱溶剂(天津四友和天津康科德等);其他试剂均为重蒸工业试剂。
1.3 样品提取和分离人工诱导的海南血竭(600.0 g)干燥后粉碎,用体积分数为95%的乙醇加热回流提取3次,每次分别加热回流3、2、1 h,过滤,合并乙醇提取液, 减压浓缩至无醇味得红色粗浸膏,将粗浸膏分散于去离子水中成悬浊液,分别用石油醚、乙酸乙酯和正丁醇各萃取3次,分别减压浓缩得浸膏。乙酸乙酯萃取物(100.0 g)经减压硅胶柱色谱,以石油醚-乙酸乙酯(10:1~1:10,V/V, 下同)梯度洗脱,经TLC检测,合并相同部分,得12个组份(Fr.1~Fr.12)。
Fr.8 (9.0 g)经Sephadex LH-20凝胶柱色谱,以甲醇洗脱得10个组份Fr.8A~Fr.8J。Fr.8B (1.77 g)经Sephadex LH-20凝胶柱色谱,以乙醇洗脱得2个组份Fr.8B1和Fr.8B2;Fr.8B1 (1.34 g)经硅胶柱色谱,以氯仿-甲醇(100:1)洗脱得Fr.8B1A~Fr.8B1E;Fr.8B1C (379.7 mg)经Sephadex LH-20凝胶柱色谱,以甲醇洗脱,再经硅胶色谱柱,以石油醚-乙酸乙酯(7:3~2:1)梯度洗脱得化合物10 (4.1 mg); Fr.8B1D (72.4 mg)经Sephadex LH-20凝胶柱色谱和硅胶色谱柱,再经半制备高效液相色谱(C18柱;75%甲醇水;流速4 mL min–1;检测波长200,254 nm)恒梯度洗脱得到化合物2 (4.0 mg,保留时间4.5 min); Fr.8B1E (26.1 mg)经Sephadex LH-20凝胶柱色谱(甲醇)和硅胶色谱柱(石油醚:氯仿:丙酮=5:5:1)恒梯度洗脱得到化合物1 (2.0 mg)。
Fr.9 (10.7 g)经RP-18反相硅胶柱色谱,以甲醇-水(30%~100%)梯度洗脱得到12个组份Fr.9A~ Fr.9L。Fr.9D (369.0 mg)经Sephadex LH-20柱色谱(甲醇)分离得到7个组份Fr.9D1~Fr.9D7;Fr.9D2 (209.8 mg)经硅胶柱色谱以石油醚-乙酸乙酯(2:1)洗脱得化合物9 (8.5 mg);Fr.9D3(148.6 mg)经硅胶柱色谱,再经半制备高效液相色谱(C18柱;40%甲醇水;流速4 mL min–1;检测波长210,230 nm)恒梯度洗脱得到化合物4 (2.5 mg,保留时间35.5 min); Fr.9D5 (163.1 mg)经硅胶柱色谱以石油醚-乙酸乙酯(13:1~6:1)梯度洗脱得化合物5 (2.5 mg)、6 (4.4 mg)和7 (2.0 mg);Fr.9D7 (41.7 mg)经Sephadex LH-20柱色谱(氯仿:甲醇=1:1)和硅胶柱色谱(氯仿:甲醇=100:1)洗脱得化合物3 (3.6 mg)。Fr.9E (410.9 mg)经Sephadex LH-20柱色谱(甲醇)和硅胶柱色谱(石油醚:氯仿:甲醇=5:5:0.1),再经半制备高效液相色谱(C18柱;55%甲醇水;流速4 mL min–1;检测波长200,220 nm)恒梯度洗脱得到化合物8 (1.4 mg, 保留时间14.8 min)。
1.4 结构鉴定Socotrin-4'-ol (1) 白色无定型粉末, 分子式C31H30O6; ESIMS m/z: 521.3 [M + Na]+; 1H NMR (500 MHz, CD3OD): δH 4.15 (1H, t, J = 7.4 Hz, H-γ), 2.10 (2H, m, H-α), 2.42 (2H, m, H-β), 4.85 (1H, m, H-2), 2.03 (1H, m, H-3a), 1.93 (1H, m, H-3b), 2.83 (1H, m, H-4a), 2.62 (1H, m, H-4b), 6.80 (1H, s, H-5), 6.20 (1H, s, H-8), 7.20 (2H, d, J = 8.7 Hz, H-2', 6'), 6.75 (2H, d, J = 8.7 Hz, H-3', 5'), 6.34 (1H, d, J = 2.2 Hz, H-3"), 6.24 (1H, dd, J = 8.1, 2.3 Hz, H-5"), 6.79 (1H, d, J = 8.0 Hz, H-6"), 7.06 (2H, d, J = 8.6 Hz, H-2‴, 6‴), 6.65 (2H, d, J = 8.5 Hz, H-3‴, 5‴), 3.73 (3H, s, 2"-OMe); 13C NMR (125 MHz, CD3OD): δC 79.0 (C- 2), 31.6 (C-3), 25.8 (C-4), 113.8 (C-10), 129.4 (C-5), 126.4 (C-6), 154.8 (C-7), 103.8 (C-8), 154.1 (C-9), 134.4 (C-1'), 128.3 (C-2', C-6'), 116.0 (C-3', 5'), 123.2 (C-1"), 159.7 (C-2"), 99.7 (C-3"), 157.6 (C-4"), 107.5 (C-5"), 131.0 (C-6"), 138.3 (C-1‴), 130.1 (C-2‴, 6‴), 115.7 (C-3‴, 5‴), 43.2 (C-γ), 37.3 (C-α), 29.6 (C-β), 156.0 (C-4‴), 55.6 (2"-OMe)。以上核磁数据与文献[8]一致,确定化合物为socotrin-4'-ol。
Homoisosocotrin-4'-ol (2) 白色无定型粉末, 分子式C32H32O6; ESIMS m/z: 535.3 [M + Na]+, 511.2 [M-H]–; 1H NMR (500 MHz, CD3OD): δH 4.12 (1H, t, J = 7.8 Hz, H-γ), 2.00 (2H, m, H-α), 2.38 (2H, m, H-β), 4.01 (1H, dd, J = 8.9, 1.8 Hz, H-2a), 3.69 (1H, m, H-2b), 2.12 (1H, m, H-3), 2.35 (1H, dd, J = 15.9, 9.1 Hz, H-4a), 2.62 (1H, m, H-4b), 6.76 (1H, s, H-5), 6.14 (1H, s, H-8), 2.51 (2H, m, H-9), 6.98 (2H, d, J = 8.4 Hz, H-2', 6'), 6.68 (2H, d, J = 8.5 Hz, H-3', 5'), 6.34 (1H, d, J = 2.3 Hz, H-3"), 6.24 (1H, dd, J = 8.0, 2.3 Hz, H-5"), 6.79 (1H, d, J = 8.0 Hz, H-6"), 7.03 (2H, d, J = 8.6 Hz, H-2‴, 6‴), 6.64 (2H, d, J = 8.6 Hz, H-3‴, 5‴), 3.71 (3H, s, 2"-OMe); 13C NMR (125 MHz, CD3OD): δC 70.8 (C-2), 36.0 (C-3), 31.5 (C-4), 113.3 (C-4a), 129.8 (C-5), 126.2 (C-6), 154.8 (C-7), 103.5 (C-8), 156.0 (C-8a), 38.1 (C-9), 131.9 (C-1'), 131.0 (C-2', C-6'), 116.1 (C-3', 5'), 123.2 (C-1"), 159.7 (C-2"), 99.7 (C-3"), 157.6 (C-4"), 107.5 (C-5"), 131.1 (C-6"), 138.3 (C-1‴), 130.0 (C-2‴, 6‴), 115.7 (C-3‴, 5‴), 154.1 (C-4‴), 43.2 (C-γ), 37.3 (C-α), 29.6 (C-β), 55.6 (2"-OMe)。以上数据与文献[8]一致,确定化合物为homoisosocotrin-4'-ol。
(E)-3-(3, 4-Dihydroxybenzylidene)-7-hydroxy-chroman-4-one (3) 黄色针状结晶(甲醇), 分子式C16H12O5; ESIMS m/z: 307.2 [M + Na]+, 283.1 [M-H]–; 1H NMR (500 MHz, CD3OD): δH 5.34 (2H, s, H-2), 6.28 (1H, d, J = 2.2 Hz, H-8), 6.50 (1H, dd, J = 2.2, 8.7 Hz, H-6), 6.75 (1H, dd, J = 1.7, 8.2 Hz, H-6'), 6.80 (1H, d, J = 1.7 Hz, H-2'), 6.83 (1H, d, J = 8.2 Hz, H-5'), 7.62 (1H, s, H-9), 7.77 (1H, d, J = 8.7 Hz, H-5); 13C NMR (125 MHz, CD3OD): δC 69.1 (C-2), 127.6 (C-3), 183.0 (C-4), 115.9 (C-4a), 130.7 (C-5), 112.2 (C-6), 166.7 (C-7), 103.6 (C-8), 164.8 (C-8a), 138.5 (C-9), 129.5 (C-1'), 118.3 (C-2'), 146.6 (C-3'), 148.8 (C-4'), 116.6 (C-5'), 124.6 (C-6')。根据HMBC谱和文献[9], 确定化合物为(E)-3-(3, 4-dihydroxybenzy- lidene)-7-hydroxy-chroman-4-one。
5-hydroxy-7-methoxy-3-(4'-hydroxybenzyl)-4-chromanone (4) 白色无定型粉末, 分子式C16H12O5; ESIMS m/z: 323.2 [M + Na]+, 299.0 [M-H]–; 1H NMR (500 MHz, CD3OD): δH 4.17 (1H, dd, J = 3.3, 11.3 Hz, H-2a), 4.01 (1H, dd, J = 5.6, 11.3 Hz, H-2b), 2.58 (1H, m, H-3), 5.88 (1H, d, J = 2.1 Hz, H- 6), 6.00 (1H, d, J = 2.1 Hz, H-8), 2.99 (1H, m, H-9a), 2.58 (1H, m, H-9b), 7.03 (2H, d, J = 8.5 Hz, H-2', 6'), 6.70 (2H, d, J = 8.5 Hz, H-3', 5'); 13C NMR (125 MHz, CD3OD): δC 69.6 (C-2), 50.0 (C-3), 193.8 (C-4), 103.0 (C-4a), 167.3 (C-5), 97.3 (C-6), 164.6 (C-7), 94.9 (C-8), 166.5 (C-8a), 33.6 (C-9), 130.7 (C-1'), 131.1 (C-2', 6'), 116.3 (C-3', 5'), 157.1 (C-4')。以上数据与文献[10]报道基本一致, 确定化合物为5-hydroxy- 7-methoxy-3-(4'-hydroxybenzyl)-4-chromanone。
3-去氧苏木查耳酮 (5) 黄色无定型粉末, 分子式C16H14O4; ESIMS m/z: 293.3 [M + Na]+, 269.1 [M-H]–; 1H NMR (500 MHz, CD3OD): δH 7.39 (1H, d, J = 15.7 Hz, H-2α), 7.54 (1H, d, J = 15.7 Hz, H-3β), 6.49 (1H, d, J = 2.1 Hz, H-3'), 6.43 (1H, dd, J = 2.1, 8.5 Hz, H-5'), 7.56 (1H, d, J = 8.5 Hz, H-6'), 7.49 (2H, dd, J = 8.6 Hz, H-2, 6), 6.80 (2H, dd, J = 8.6 Hz, H-3, 5), 3.87 (3H, s, 2'-OCH3); 13C NMR (125 MHz, CD3OD): δC 125.1 (C-2α), 144.2 (C-3β), 193.1 (C-1), 121.8 (C-1'), 162.5 (C-2'), 100.1 (C-3'), 164.6 (C-4'), 108.9 (C-5'), 133.7 (C-6'), 128.0 (C-1), 131.4 (C-2, 6), 116.9 (C-3, 5), 161.2 (C-4), 56.1 (2'-OCH3)。以上数据与文献[11]报道一致,故确定化合物为3-去氧苏木查耳酮。
苏木查耳酮 (6) 黄色无定型粉末, 分子式C16H14O5; ESIMS m/z: 309.3 [M + Na]+, 285.1 [M-H]–; 1H NMR (500 MHz, CD3OD): δH 7.34 (1H, d, J = 15.6 Hz, H-2α), 7.47 (1H, d, J = 15.6 Hz, H-3β), 6.49 (1H, d, J = 2.1 Hz, H-3'), 6.43 (1H, dd, J = 2.1, 8.5 Hz, H-5'), 7.55 (1H, d, J = 8.5 Hz, H-6'), 7.08 (1H, d, J = 1.9 Hz, H-2), 6.77 (2H, d, J = 8.2 Hz, H-5), 6.96 (1H, dd, J = 1.9, 8.2 Hz, H-6), 3.87 (3H, s, 2'-OCH3); 13C NMR (125 MHz, CD3OD): δC 125.1 (C-2α), 144.6 (C-3β), 193.1 (C-1), 121.8 (C-1'), 162.5 (C-2'), 100.1 (C-3'), 164.6 (C-4'), 108.9 (C-5'), 133.7 (C-6'), 128.6 (C-1), 115.2 (C-2), 146.8 (C-3), 149.6 (C-4), 116.6 (C-5), 123.3 (C-6), 56.1 (2'-OCH3)。以上数据与文献[11]报道一致,故确定化合物为苏木查耳酮。
7, 4'-二羟基黄酮 (7) 黄色无定型粉末, 分子式C15H10O4; ESIMS m/z: 277.2 [M + Na]+, 531.3 [2M + Na]+; 1H NMR (500 MHz, DMSO-d6): δH 7.89 (2H, d, J = 8.8 Hz, H-2', 6'), 7.83 (1H, d, J = 8.7 Hz, H-5), 6.94 (1H, d, J = 2.2 Hz, H-8), 6.90 (2H, d, J = 8.8 Hz, H-3', 5'), 6.87 (1H, dd, J = 8.7, 2.2 Hz, H-6), 6.70 (1H, s, H-3); 13C NMR (125 MHz, DMSO-d6): δC 162.5 (C-2), 104.4 (C-3), 176.3 (C-4), 126.4 (C-5), 114.9 (C-6), 162.4 (C-7), 102.5 (C-8), 157.4 (C-9), 115.9 (C-10), 121.8 (C-1'), 128.1 (C-2', 6'), 115.9 (C-3', 5'), 160.7 (C-4')。以上数据与文献[12]报道一致,故确定化合物为7, 4'-二羟基黄酮。
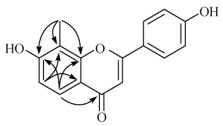
7, 4'-二羟基-8-甲基黄酮 (8) 黄色无定型粉末,根据HR-ESI-MS中的分子离子峰(m/z 269.080 8 [M + H]+, C16H13O4计算值269.081 4),结合13C NMR (DEPT)得出其分子式为C16H12O4。通过1H和13C NMR谱数据可以推断该化合物为黄酮类化合物。1H NMR谱图显示该化合物有1个AA'BB'偶合系统[δH 7.85 (2H, d, J = 8.8 Hz, H-2', 6'), 6.89 (2H, d, J = 8.8 Hz, H-3', 5')],表明该化合物B环为1个对羟基取代的苯环;1个AB偶合系统[δH 7.75 (1H, d, J = 8.8 Hz, H-5), 6.84 (1H, d, J = 8.8 Hz, H-6)],可以推断出A环为1个四取代的苯环。根据HMBC相关信号显示(图 1),A环上的质子信号δH 6.84 (H-6)与δC 113.1 (C-8), 116.1 (C-4a)的相关,质子信号δH 7.75 (H-5)与δC 180.8 (C-4),157.8 (C-7)和164.9 (C-8a)的相关以及甲基信号δH 2.36 (-CH3)与δC 113.1 (C-8),157.8 (C-7)和164.9 (C-8a)相关,表明甲基与C-8相连, 其中1个羟基位于C-7位上。综上所述该化合物鉴定为7, 4'-二羟基-8-甲基黄酮。通过HSQC和HMBC谱(图 1)分析, 归属了该化合物的1H NMR和13C NMR数据。1H NMR (500 MHz, CD3OD): δH 7.85 (2H, d, J = 8.8 Hz, H-2', 6'), 7.75 (1H, d, J = 8.8 Hz, H-5), 6.89 (2H, d, J = 8.8 Hz, H-3', 5'), 6.84 (1H, d, J = 8.8 Hz, H-6), 6.61 (1H, s, H-3), 2.36 (3H, s, 8-CH3); 13C NMR (125 MHz, CD3OD): δC 165.8 (C-2), 104.3 (C-3), 180.8 (C-4), 116.1 (C-4a), 124.2 (C-5), 116.2 (C-6), 157.8 (C-7), 113.1 (C-8), 164.9 (C-8a), 123.6 (C-1'), 129.3 (C-2', 6'), 117.3 (C-3', 5'), 163.3 (C-4')。
|
图 1 化合物8的关键HMBC信号 Fig. 1 Key HMBC signals of compound 8 |
丁香树脂醇 (9) 白色无定型粉末, 分子式C22H26O8; ESIMS m/z: 441.3 [M + Na]+, 417.1 [M-H]–; 1H NMR (500 MHz, CD3OD): δH 3.11 (2H, m, H-8, 8′), 3.81 (12H, s, 4×OCH3), 4.23 (2H, m, H-9a, 9′a), 3.85 (2H, m, H-9b, 9′b), 4.69 (2H, d, J = 4.4 Hz, H-7, 7′), 6.63 (4H, s, H-2, 6, 2′, 6′); 13C NMR (125 MHz, CD3OD): δC 55.5 (C-8, 8′), 56.8 (4×OCH3), 72.8 (C-9, 9′), 87.6 (C-7, 7′), 104.4 (C-2, 2′, 6, 6′), 133.1 (C-1, 1′), 136.2 (C-4, 4′), 149.3 (C-3, 3′, 5, 5′)。以上数据与文献[13]报道一致,故确定化合物为丁香树脂醇。
邻苯二甲酸二(2-乙基己基)酯 (10) 黄色油状物, 分子式C24H38O4; ESIMS m/z: 413.5 [M + Na]+, 389.1 [M-H]–; 1H NMR (CDCl3, 500 MHz): δH 7.72 (2H, m, H-3, 6), 7.54 (2H, m, H-4, 5), 4.23 (2H, t, J = 6.0 Hz, H-1′), 1.69 (2H, m, H-2′), 1.43 (2H, m, H-1″), 1.38 (2H, m, H-3′), 1.33 (2H, m, H-4′), 1.31 (2H, m, H-5′), 0.91 (3H, t, J = 7.4 Hz, H-2″), 0.90 (3H, t, J = 7.3 Hz, H-6′),13C NMR (125 MHz, CDCl3): δC 167.9 (COO-), 132.6 (C-1, 2), 131.0 (C-4, 5), 128.9 (C- 3, 6), 68.3 (C-1′), 38.9 (C-2′), 30.5 (C-3′), 29.1 (C-4′), 23.1 (C-5′), 14.2 (C-6′), 23.9 (C-1″), 11.1 (C-2″)。以上波谱数据与文献[14]报道基本一致,故确定化合物为邻苯二甲酸二(2-乙基己基)酯。
1.5 抑菌活性测试采用滤纸片琼脂扩散法[15]测定化合物1~10的抗菌活性。耐甲氧西林金黄色葡萄球菌(methicillin- resistant Staphylococcus aureus, MRSA)在NA培养基(牛肉膏3.0 g、琼脂20.0 g、蛋白胨10.0 g和NaCl 5.0 g,pH为7.0~7.2)上培养。将MRSA分别制成一定浓度的菌悬液(105~107 cfu mL–1), 用棉签均匀涂布于供试无菌平板,制成含菌平板。将化合物1~10和万古霉素分别配成浓度为1 mg mL–1的溶液,取10 μL分别滴加于直径为6 mm的灭菌滤纸片上,待溶剂挥干后将滤纸片置于含菌平板上, 每个样品平行3次,以万古霉素为阳性对照, 37℃恒温条件下培养。24 h后测量并记录抑菌圈直径。结果表明,化合物7和8对耐甲氧西林金黄色葡萄球菌具有生长抑制作用,其抑菌圈直径分别为12.0和13.0 mm, 阳性对照万古霉素的抑菌圈直径均为21.0 mm。
采用微量稀释法[16],进一步测定了化合物7和8对耐甲氧西林金黄色葡萄球菌生长抑制活性, 其MIC值分别为61.52和7.29 μmol L–1,万古霉素的MIC为0.67 μmol L–1。
2 结果和讨论本研究采用多种分离手段和技术,从人工诱导的海南血竭中分离鉴定了10个化合物,分别为soco- trin-4'-ol (1)、homoisosocotrin-4'-ol (2)、(E)-3-(3, 4- dihydroxybenzylidene)-7-hydroxy-chroman-4-one (3)、5-hydroxy-7-methoxy-3-(4'-hydroxybenzyl)-4-chro- manone (4)、3-deoxysappanch alcone (5)、sappan- chalcone (6)、7, 4'-二羟基黄酮(7)、7, 4'-二羟基-8-甲基黄酮(8)、丁香树脂醇(9)和邻苯二甲酸二(2-乙基己基)酯(10)。采用滤纸片琼脂扩散法测定化合物1~10的抗菌活性,结果表明,化合物7和8对耐甲氧西林金黄色葡萄球菌具有生长抑制作用, 其MIC值分别为61.52和7.29 μmol L–1。
化合物1和2已从龙血树属植物Dracaena cinnabari所产血竭中分离鉴定[8],化合物7从剑叶龙血树所产血竭中分离鉴定[12]。化合物3~6为首次从人工血竭中分离鉴定,但是其基本骨架与天然血竭保持一致,只是其取代基发生了变化,这进一步说明通过输液法诱导的人工血竭化学成分与天然血竭基本一致。
| [1] |
GUPTA D, BLEAKLEY B, GUPTA R K. Dragon's blood:Botany, chemistry and therapeutic uses[J]. J Ethnopharmacol, 2008, 115(3): 361-380. DOI:10.1016/j.jep.2007.10.018 |
| [2] |
HE L, WANG Z H, TU P F, et al. Advances in study on chemical constituents and pharmacological activities in plants of Dracaena Vand. ex L.[J]. Chin Trad Herb Drugs, 2004, 35(2): 221-228. 何兰, 王竹红, 屠鹏飞, 等. 龙血树属植物化学成分及药理活性研究进展[J]. 中草药, 2004, 35(2): 221-228. DOI:10.3321/j.issn:0253-2670.2004.02.043 |
| [3] |
LUO Y, WANG H, ZHAO Y X, et al. Cytotoxic and antibacterial flavonoids from dragon's blood of Dracaena cambodiana[J]. Planta Med, 2011, 77(18): 2053-2056. DOI:10.1055/s-0031-1280086 |
| [4] |
ZHENG D J, YUN Y, WU Y J, et al. Geographical distribution of wild Dracaena cambodiana in China and its relationship with hydrothermal factors[J]. J Trop Subtrop Bot, 2012, 20(4): 326-332. 郑道君, 云勇, 吴宇佳, 等. 海南龙血树野生资源分布及其与水热关系的分析[J]. 热带亚热带植物学报, 2012, 20(4): 326-332. DOI:10.3969/j.issn.1005-3395.2012.04.002 |
| [5] |
JIANG H M, WANG H, WANG J, et al. Antibacterial components from artificially induced dragon's blood of Dracaena cambodiana[J]. China J Chin Mat Med, 2015, 40(20): 4002-4006. 蒋和梅, 王辉, 王军, 等. 人工诱导海南龙血树所产血竭的抗菌活性成分研究[J]. 中国中药杂志, 2015, 40(20): 4002-4006. |
| [6] |
WANG H, JIANG H M, LI F X, et al. Flavonoids from artificially induced dragon's blood of Dracaena cambodiana[J]. Fitoterapia, 2017, 121: 1-5. DOI:10.1016/j.fitote.2017.06.019 |
| [7] |
LI F X, WANG H, GAI C J, et al. Three new flavanoids from artificially induced dragon's blood of Dracaena cambodiana[J]. J Asian Nat Prod Res, 2018, 20(1): 55-61. DOI:10.1080/10286020.2017.1322583 |
| [8] |
MASAOUD M, HIMMELREICH U, RIPPERGER H, et al. New biflavonoids from dragon's blood of Dracaena cinnabari[J]. Planta Med, 1995, 61(4): 341-344. DOI:10.1055/s-2006-958096 |
| [9] |
CHEN Y P, LIU L, ZHOU Y H, et al. Chemical constituents from sappan lignum[J]. J Chin Pharm Sci, 2008, 17(1): 82-86. |
| [10] |
MUTANYATTA J, MATAPA B G, SHUSHU D D, et al. Homoiso- flavonoids and xanthones from the tubers of wild and in vitro regenerated Ledebouria graminifolia and cytotoxic activities of some of the homoisoflavonoids[J]. Phytochemistry, 2003, 62(5): 797-804. DOI:10.1016/S0031-9422(02)00622-2 |
| [11] |
ESCOBAR-RAMOS A, LOBATO-GARCíA C E, ZAMILPA A, et al. Homoisoflavonoids and chalcones isolated from Haematoxylum campechianum L., with spasmolytic activity[J]. Molecules, 2017, 22(9): 1405. DOI:10.3390/molecules22091405 |
| [12] |
TU P F, TAO J, HU Y Q, et al. Flavones from the wood Dracaena conchinchinensis[J]. Chin J Nat Med, 2003, 1(1): 27-29. 屠鹏飞, 陶晶, 胡迎庆, 等. 龙血竭黄酮类成分研究[J]. 中国天然药物, 2003, 1(1): 27-29. |
| [13] |
ZHAO L J, WANG Y W, LI Z F, et al. Isolation and identification of chemical constituents from Sabia parviflora[J]. Chin Trad Herb Drugs, 2018, 49(3): 544-548. 赵兰君, 王玉伟, 李志峰, 等. 小花清风藤化学成分的分离与鉴定[J]. 中草药, 2018, 49(3): 544-548. DOI:10.7501/j.issn.0253-2670.2018.03.006 |
| [14] |
HUANG M X, CAI Y J, LIU S B, et al. Study on the chemical constituents of Clinacanthus nutans branches and leaves and its antitumor activity in vitro[J]. China Pharm, 2017, 28(7): 895-898. 黄茂莘, 蔡杨靖, 刘寿柏, 等. 忧遁草枝叶的化学成分及体外抗肿瘤活性研究[J]. 中国药房, 2017, 28(7): 895-898. DOI:10.6039/j.issn.1001-0408.2017.07.09 |
| [15] |
XU S Y, BIAN R L, CHEN X. Experimental Methodology of Pharmacology[M]. 3rd ed. Beijing: People's Sanitation Press, 1982: 1-651. 徐叔云, 卞如濂, 陈修. 药理实验方法学[M]. 第3版. 北京: 人民卫生出版社, 1982: 1-651. |
| [16] |
SUN F H, LONG N N, WANG X M, et al. In vitro antibacterial activity of geraniol against methicillin-resistant Staphylococcus aureus[J]. Chin J Antibiot, 2018, 43(7): 921-926. 孙丰慧, 龙娜娜, 王雪梅, 等. 香叶醇体外抗MRSA活性研究[J]. 中国抗生素杂志, 2018, 43(7): 921-926. DOI:10.3969/j.issn.1001-8689.2018.07.025 |
 2019, Vol. 27
2019, Vol. 27



