美人蕉科(Cannaceae)美人蕉属(Canna)植物原产美洲的热带及亚热带地区,我国各地均有栽培, 由于其叶片粗犷、花色娇艳、花期长、易于栽培等优点,被广泛应用于园林绿化,具有很高的观赏价值[1];根茎具有清热利湿、舒筋活络的作用,主治黄疸肝炎、风湿麻木、外伤出血、跌打、子宫下垂、心气痛等[2];花具有止血功效[3]。美人蕉(C. indica)的醇提物能抑制脂多糖诱导的炎症因子产生,并通过控制丝裂原活化蛋白激酶通路来抑制高糖诱导的炎症因子表达[4],还有镇痛和驱虫活性[5]。目前对美人蕉的研究主要集中在植物修复、环境治理及其花色素的提取应用上,对化学成分的报道较少。从美人蕉根茎中分离鉴定了棕榈酸、棕榈酸1-单甘油酯、β-谷甾醇、胡萝卜甙和对映-11α-羟基-15-酮-贝壳杉-16-烯-19-甲酸[6],从花中获得了6个花青苷类化合物[7]。大花美人蕉(C. generalis)在园林上应用较广,但其化学成分的研究未见报道。本研究以大花美人蕉根为材料,从其乙醇提取物中分离鉴定了8个化合物,且均为首次从该植物中得到。本文报道这些化合物的分离鉴定,为大花美人蕉的开发利用提供科学依据。
1 材料和方法 1.1 材料大花美人蕉(Canna generalis)于2015年5月购于湖北省武汉市汉口北的四季美花木市场,产地为武汉市蔡甸区,由中国科学院华南植物园童毅华助理研究员鉴定。
薄层层析(TLC)板为烟台江友硅胶开发公司HSGF254硅胶板;柱层析用硅胶(100~200目或200~ 300目)为青岛海洋化工有限公司产品;柱层析反相硅胶Chromatorex C18 (MB100-40, 粒径40~75 μm)为日本富士化学公司产品。显色方法为紫外(254 nm)和喷洒10%硫酸-乙醇溶液并烘烤显色。
1.2 仪器赛默飞世尔科技有限公司的UltiMate 3000液相色谱仪,检测器为VWD-3100紫外检测器,色谱柱为AcclaimTM 120 C18 (4.6 mm×150 mm, 5 μm); 德国IKA集团RV10基本型旋转蒸发仪,配以武汉科尔仪器设备有限公司SHZ-D(Ⅲ)循环水式真空泵和上海卫凯仪器设备有限公司DLSB-5/20低温冷却循环泵;1H NMR和13C NMR采用美国安捷伦科技有限公司的Agilent 600 MHz DD2核磁共振波谱仪,以氘代溶剂残留峰为参照;Agilent 6120 LC/MS液质联用仪,电喷雾(ESI)电离源。
1.3 提取和分离去除大花美人蕉茎叶及须根,将根切片晾干粉碎,得到粉末2.5 kg;用95%乙醇溶液浸泡提取3次,每次2 d;再用80%乙醇溶液浸泡提取3次, 每次2 d。提取液合并后减压浓缩得无乙醇溶液2 L, 依次用石油醚、乙酸乙酯萃取,减压浓缩后得到石油醚萃取物(24.2 g)及乙酸乙酯萃取物(15.7 g)。
乙酸乙酯萃取物(15.7 g)进行正相硅胶柱层析, 以氯仿-甲醇[10:0~7:3, V/V (下同)]梯度洗脱,收集流份,TLC检查后合并为6个组分(E1~E6)。E1 (2.6 g)经正相硅胶柱层析,以石油醚-乙酸乙酯(8:2~7:3)洗脱,TLC检查后合并为4个亚组分(E1-1~ E1-4)。E1-3经反相硅胶柱层析,以甲醇-水(4:6~ 7:3)梯度洗脱,得到化合物1 (5 mg)。E2 (1.7 g)经正相硅胶柱层析,以石油醚-乙酸乙酯(8:2)洗脱, TLC检查后合并为5个亚组分(E2-1~E2-5)。E2-1经反相硅胶柱层析,以甲醇-水(3:7~6:4)梯度洗脱,得到化合物2 (11.1 mg)和3(15.3 mg)。
石油醚萃取物(24.2 g)经正相硅胶柱层析,以石油醚-乙酸乙酯(10:0~65:35)梯度洗脱,收集流份,TLC检查后合并为10个组分(P1~P10)。P3 (2.4 g)经反相硅胶柱层析,以甲醇-水(5:5~8:2)梯度洗脱,TLC检查后合并为3个亚组分(P3-1~P3-3)。P3-2经正相硅胶柱层析,以石油醚-乙酸乙酯(95:5)洗脱,TLC检查后合并为2个亚组分(P3-2-1~ P3-2-2)。P3-2-2经HPLC制备,以甲醇-水(95:5)为流动相,流速1 mL min–1,得到化合物4(tR=21 min, 2.7 mg)和5(tR=24 min, 2.7 mg)。P4 (1.8 g)经反相硅胶柱层析,以甲醇-水(8:2~9:1)梯度洗脱,TLC检查后合并为7个亚组分(P4-1~P4-7)。P4-4经正相硅胶柱层析,以石油醚-乙酸乙酯(98:2)洗脱,再经反相硅胶柱层析,以甲醇-水(9:1~10:0)梯度洗脱,得到化合物6(12 mg)。P4-5经正相硅胶柱层析,以石油醚-乙酸乙酯(95:5)洗脱,再经反相硅胶柱层析, 以甲醇-水(95:5~10:0)梯度洗脱,得到化合物7 (260 mg)。P4-6经正相硅胶柱层析,以石油醚-乙酸乙酯(95:5)洗脱,再经反相硅胶柱层析,以甲醇-水(95:5~10:0)梯度洗脱,得到化合物8(2.0 mg)。各化合物的结构见图 1。
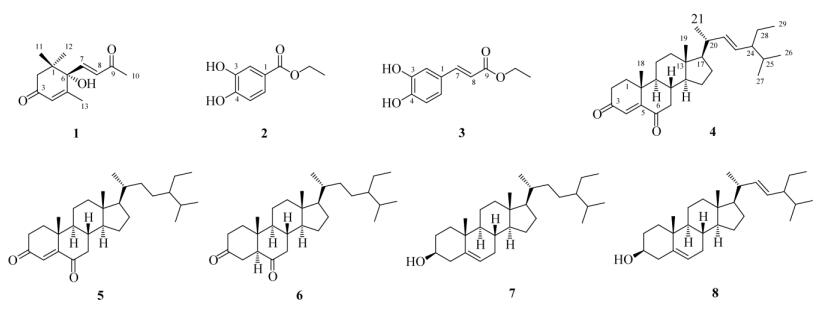
|
图 1 化合物1~8的结构 Fig. 1 Structures of compounds 1–8 |
化合物1 白色粉末, 分子式C13H18O3; ESI- MS m/z: 223 [M + H]+, 261 [M + K]+; 1H NMR (CD3OD, 600 MHz): δ 6.99 (1H, d, J = 15.8 Hz, H-7), 6.44 (1H, d, J= 15.8 Hz, H-8), 5.93 (1H, s, H-4), 2.60 (1H, d, J = 17.1 Hz, Ha-2), 2.31 (3H, s, H3-10), 2.28 (1H, d, J= 17.1 Hz, Hb-2), 1.90 (3H, d, J = 1.3 Hz, H3-13), 1.06 (3H, s, H3-11), 1.02 (3H, s, H3-12); 13C NMR (CD3OD, 150 MHz): δ 42.6 (C-1), 50.5 (C-2), 200.4 (C-3), 128.0 (C-4), 164.7 (C-5), 80.0 (C-6), 148.3 (C-7), 131.7 (C-8), 200.7 (C-9), 27.7 (C-10), 23.5 (C-11), 24.7 (C-12), 19.2 (C-13)。以上数据与文献[8]报道一致,故鉴定为去氢催吐萝芙木醇。
化合物2 黄色粉末, 分子式C9H10O4;ESI- MS m/z: 183 [M + H]+; 1H NMR (CD3OD, 600 MHz): δ 7.42 (1H, d, J = 2.0 Hz, H-2), 7.41 (1H, dd, J = 8.1, 2.0 Hz, H-6), 6.80 (1H, d, J = 8.1 Hz, H-5), 4.29 (2H, q, J = 7.1 Hz, OCH2), 1.35 (3H, t, J = 7.1 Hz, CH3); 13C NMR (CD3OD, 150 MHz): δ 122.9 (C-1), 115.8 (C-2), 146.1 (C-3), 151.6 (C-4), 117.4 (C-5), 123.5 (C-6), 168.4 (C=O), 61.6 (OCH2), 14.6 (CH3)。以上数据与文献[9]报道一致,故鉴定为3, 4-二羟基苯甲酸乙酯。
化合物3 褐色粉末, 分子式C11H12O4; ESI- MS m/z: 209 [M + H]+; 1H NMR (CD3OD, 600 MHz): δ 7.53 (1H, d, J= 15.9 Hz, H-7), 7.03 (1H, d, J =1.8 Hz, H-2), 6.94 (1H, dd, J = 8.1, 1.6 Hz, H-6), 6.78 (1H, d, J= 8.2 Hz, H-5), 6.24 (1H, d, J = 15.9 Hz, H-8), 4.21 (2H, q, J = 7.1 Hz, OCH2), 1.31 (3H, t, J = 7.1 Hz, CH3); 13C NMR (CD3OD, 150 MHz): δ 146.7 (C-1, 3), 127.7 (C-2), 149.5 (C-4), 122.9 (C-5), 116.5 (C-6), 115.2 (C-7), 115.1 (C-8), 169.3 (C-9), 61.4 (OCH2), 14.6 (CH3)。以上数据与文献[10]报道一致,故鉴定为咖啡酸乙酯。
化合物4 白色结晶, 分子式C29H44O2; 1H NMR (CDCl3, 600 MHz): δ 6.17 (1H, s, H-4), 5.15 (1H, dd, J = 15.2, 8.6 Hz, H-23), 5.04 (1H, dd, J = 15.2, 8.7 Hz, H-22), 1.17 (3H, s, H3-19), 1.03 (3H, d, J = 6.6 Hz, H3-21), 0.85 (3H, d, J = 6.2 Hz, H3-27), 0.80 (3H, d, J = 6.6 Hz, H3-26), 0.74 (3H, s, H3-18); 13C NMR (CDCl3, 150 MHz): δ 35.7 (C-1), 34.1 (C-2), 202.5 (C-3), 125.6 (C-4), 161.2 (C-5), 199.6 (C-6), 47.0 (C-7), 34.4 (C-8), 51.2 (C-9), 40.0 (C-10), 21.0 (C-11), 39.2 (C-12), 42.6 (C-13), 55.9 (C-14), 24.2 (C-15), 28.8 (C-16), 56.8 (C-17), 12.2 (C-18), 17.7 (C-19), 40.5 (C-20), 21.2 (C-21), 137.9 (C-22), 129.9 (C-23), 51.4 (C-24), 32.0 (C-25), 19.1 (C-26), 21.3 (C-27), 25.5 (C-28), 12.4 (C-29)。以上数据与文献[11]报道一致,故鉴定为豆甾-4, 22-二烯-3, 6-二酮。
化合物5 白色结晶, 分子式C29H46O2; 1H NMR (CDCl3, 600 MHz): δ 6.17 (1H, s, H-4), 2.68 (1H, dd, J = 16.0, 4.1 Hz, Hb-7), 2.52 (1H, dd, J = 14.7, 5.1 Hz, Hb-2), 2.47 (1H, m, Ha-2), 2.03 (1H, dd, J = 16.0, 12.4 Hz, Ha-7), 1.16 (3H, s, H3-19), 0.93 (3H, d, J = 6.5 Hz, H3-21), 0.85 (3H, t, J = 7.5 Hz, H3- 29), 0.84 (3H, d, J = 6.8 Hz, H3-26), 0.82 (3H, d, J = 6.8 Hz, H3-27), 0.72 (3H, s, H3-18); 13 C NMR (CDCl3, 150 MHz): δ 35.7 (C-1), 34.1 (C-2), 199.6 (C-3), 125.6 (C-4), 161.2 (C-5), 202.5 (C-6), 47.0 (C-7), 34.4 (C-8), 51.1 (C-9), 40.0 (C-10), 21.0 (C-11), 39.3 (C-12), 42.7 (C-13), 56.7 (C-14), 24.1 (C-15), 28.2 (C-16), 56.0 (C-17), 12.0 (C-18), 17.7 (C-19), 36.2 (C-20), 18.9 (C-21), 34.0 (C-22), 26.2 (C-23), 46.0 (C-24), 29.3 (C-25), 20.0 (C-26), 19.2 (C-27), 23.2 (C-28), 12.1 (C-29)。以上数据与文献[12]报道一致, 故鉴定为豆甾-4-烯-3, 6-二酮。
化合物6 白色粉末, 分子式C29H48O2; 1H NMR (CDCl3, 600 MHz): δ 0.95 (3H, s, H3-19), 0.92 (3H, d, J = 6.5 Hz, H3-21), 0.85 (3H, d, J = 7.2 Hz, H3-26), 0.83 (3H, t, J = 6.8 Hz, H3-29), 0.81 (3H, d, J = 6.8 Hz, H3-27), 0.69 (3H, s, H3-18); 13C NMR (CDCl3, 150 MHz): δ38.3 (C-1), 37.5 (C-2), 211.5 (C-3), 37.2 (C-4), 57.7 (C-5), 209.3 (C-6), 46.8 (C-7), 38.2 (C-8), 53.6 (C-9), 41.4 (C-10), 21.8 (C-11), 39.5 (C-12), 43.2 (C-13), 56.8 (C-14), 24.2 (C-15), 28.2 (C-16), 56.2 (C-17), 12.2 (C-18), 12.7 (C-19), 36.2 (C-20), 18.9 (C-21), 34.0 (C-22), 26.2 (C-23), 46.0 (C-24), 29.3 (C-25), 20.0 (C-26), 19.2 (C-27), 23.2 (C-28), 12.1 (C-29)。以上数据与文献[12]报道一致,故鉴定为豆甾烷-3, 6-二酮。
化合物7 白色粉末, 分子式C29H50O; ESI- MS m/z: 415 [M + H]+; 1H NMR (CDCl3, 600 MHz): δ 5.35 (1H, m, H-6), 3.52 (1H, m, H-3), 1.01 (3H, s, H3-19), 0.92 (3H, d, J = 6.4 Hz, H3-21), 0.85 (3H, d, J = 7.3 Hz, H3-26), 0.83 (3H, t, J = 6.8 Hz, H3-29), 0.81 (3H, d, J = 6.8 Hz, H3-27), 0.68 (3H, s, H3-18); 13C NMR (CDCl3, 150 MHz): δ37.4 (C-1), 31.8 (C-2), 72.0 (C-3), 42.4 (C-4), 140.9 (C-5), 121.9 (C-6), 32.1 (C-7, 8), 50.3 (C-9), 36.7 (C-10), 21.2 (C-11), 39.9 (C-12), 56.9 (C-14), 24.5 (C-15), 28.4 (C-16), 56.2 (C-17), 12.1 (C-18), 19.6 (C-19), 36.3 (C-20), 18.9 (C-21), 34.1 (C-22), 26.2 (C-23), 46.0 (C-24), 29.3 (C-25), 20.0 (C-26), 19.2 (C-27), 23.2 (C-28), 12.0 (C-29)。以上数据与文献[13]报道一致,故鉴定为β-谷甾醇。
化合物8 白色粉末, 分子式C29H48O; 1H NMR (CDCl3, 600 MHz): δ 5.35 (1H, s, H-6), 5.15 (1H, dd, J= 15.1, 8.7 Hz, H-22), 5.02 (1H, dd, J = 15.0, 8.9 Hz, H-23), 3.52 (1H, m, H-3); 13C NMR (CDCl3, 150 MHz): δ 37.4 (C-1), 32.1 (C-2), 72.0 (C- 3), 42.5 (C-4), 140.9 (C-5), 121.9 (C-6), 31.8 (C-7), 32.1 (C-8), 50.3 (C-9), 36.7 (C-10), 21.2 (C-11), 39.9 (C-12), 56.1 (C-14), 24.5 (C-15), 29.1 (C-16), 57.0 (C-17), 12.2 (C-18), 19.6 (C-19), 40.6 (C-20), 21.4 (C-21), 138.5 (C-22), 129.4 (C-23), 51.4 (C-24), 32.1 (C-25), 19.1 (C-26), 21.2 (C-27), 25.6 (C-28), 12.4 (C-29)。以上数据与文献[13]报道一致,故鉴定为豆甾醇。
2 结果和讨论本研究以大花美人蕉根为材料,利用硅胶柱和多种色谱方法分离鉴定了8个化合物,包括大柱烷类、酚类及甾醇类,分别为去氢催吐萝芙木醇(1)、3, 4-二羟基苯甲酸乙酯(2)、咖啡酸乙酯(3)、豆甾- 4, 22-二烯-3, 6-二酮(4)、豆甾-4-烯-3, 6-二酮(5)、豆甾烷-3, 6-二酮(6)、β-谷甾醇(7)和豆甾醇(8),所有化合物均为首次从该植物中分离得到,可为美人蕉根的开发利用提供依据。据文献报道,去氢催吐萝芙木醇(1)对小鼠腹腔巨噬细胞RAW 264.7中脂多糖诱导一氧化氮的产生具有抑制作用(IC50=24.4 μg mL–1)[14]。3, 4-二羟基苯甲酸乙酯(2)作为脯氨酰羟化酶抑制剂,在人皮肤成纤维细胞的胶原合成过程中能有效抑制脯氨酰羟化[15]。咖啡酸乙酯(3)对中国仓鼠V79细胞中H2O2诱导产生的细胞毒性及DNA单链断裂具有抑制作用[16]。豆甾-4-烯-3, 6-二酮(5)具有化感作用,当浓度为6.5 mg L–1时对铜绿微囊藻的抑制率达到48%[17]。豆甾烷-3, 6-二酮(6)在40 μg mL–1下具有显著的克服多药耐药及P-糖蛋白(MDR-Pgp)的调制活性,且存在剂量相关性[18]。β-谷甾醇(7)和豆甾醇(8)具有抗炎、抗氧化、抗肿瘤、降低胆固醇等多种药理活性[19-20]。
| [1] |
HUANG G T, OUYANG D M, XIANG Q B, et al. Studies on classification for cultivars of Canna L.[J].
J Nanjing For Univ (Nat Sci), 2005, 29(4): 20-24. 黄国涛, 欧阳底梅, 向其柏, 等. 美人蕉属品种分类研究[J]. 南京林业大学学报(自然科学版), 2005, 29(4): 20-24. DOI:10.3969/j.issn.1000-2006.2005.04.005 |
| [2] |
WU D L. Musaceae Zingiberaceae Cannaceae[M]//Flora Reipublicae Popularis Sinicae, Tomus 16(2). Beijing: Science Press, 1981: 155-157.
吴德邻.芭蕉科姜科美人蕉科[M]//中国植物志, 第16卷第2分册.北京: 科学出版社, 1981: 155-157. |
| [3] |
ZHANG L, ZHANG B E, HUANG L, et al. Hemostatic effect of Canna indica L.[J].
J Dali Univ, 2011, 10(12): 24-26. 张琳, 张柏娥, 黄丽, 等. 美人蕉花的止血作用研究[J]. 大理学院学报, 2011, 10(12): 24-26. DOI:10.3969/j.issn.1672-2345.2011.12.007 |
| [4] | CHEN H J, CHEN C N, SUNG M L, et al. Canna indica L. attenuates high-glucose-and lipopolysaccharide-induced inflammatory mediators in monocyte/macrophage[J]. J Ethnopharmacol, 2013, 148(1): 317-321. DOI:10.1016/j.jep.2013.04.037 |
| [5] | NIRMAL S A, SHELKE S M, GAGARE P B, et al. Antinociceptive and anthelmintic activity of Canna indica[J]. Nat Prod Res, 2007, 21(12): 1042-1047. DOI:10.1080/14786410701526016 |
| [6] |
TANG X Y, LIU J, ZHANG Z H, et al. Studies on the chemical constituents of Canna indica[J].
Chin Trad Herb Drugs, 1995, 26(2): 107 唐祥怡, 刘军, 张执候, 等. 美人蕉的化学成分研究[J]. 中草药, 1995, 26(2): 107 DOI:10.7501/j.issn.0253-2670.1995.2.025 |
| [7] | SRIVASTAVA J, VANKAR P S. Methylated anthocyanidin glycol-sides from flowers of Canna indica[J]. Carbohydr Res, 2010, 345(14): 2023-2029. DOI:10.1016/j.carres.2010.07.015 |
| [8] | KAI H, BABA M, OKUYAMA T. Two new megastigmanes from the leaves of Cucumis sativus[J]. Chem Pharm Bull, 2007, 55(1): 133-136. DOI:10.1248/cpb.55.133 |
| [9] |
HUANG M Z, CHEN H S, LIU J G, et al. Studies on the chemical constituents of Bidens bipinnata L.[J].
Acad J Sec Mil Med Univ, 2006, 27(8): 888-891. 黄敏珠, 陈海生, 刘建国, 等. 中药鬼针草化学成分的研究[J]. 第二军医大学学报, 2006, 27(8): 888-891. DOI:10.3321/j.issn:0258-879X.2006.08.019 |
| [10] |
QIN L H, GUO X Y, FAN M, et al. Anti-anoxic constituents from Mesona chinensis Benth.[J].
J Shenyang Pharm Univ, 2006, 23(10): 633-636. 秦立红, 郭晓宇, 范明, 等. 凉粉草中抗缺氧化学成分[J]. 沈阳药科大学学报, 2006, 23(10): 633-636. DOI:10.3969/j.issn.1006-2858.2006.10.005 |
| [11] |
JIA L, GUO M M, LI D, et al. Chemical constituents from petroleum ether portion of Abelmoschus esculentus Ⅱ[J].
China J Chin Mat Med, 2011, 36(7): 891-895. 贾陆, 郭明明, 李东, 等. 黄秋葵石油醚部位化学成分的研究Ⅱ[J]. 中国中药杂志, 2011, 36(7): 891-895. DOI:10.4268/cjcmm20110715 |
| [12] | WEI K, LI W, KOIKE K, et al. Complete 1H and 13C NMR assign-ments of two phytosterols from roots of Piper nigrum[J]. Magn Reson Chem, 2004, 42(3): 355-359. DOI:10.1002/mrc.1347 |
| [13] | CHATURVEDULA V S P, PRAKASH I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus[J]. Int Curr Pharm J, 2012, 1(9): 239-242. DOI:10.3329/icpj.v1i9.11613 |
| [14] | LEE C, LEE S, PARK S Y. A new monoterpene from the flower buds of Buddleja officinalis[J]. Nat Prod Sci, 2013, 19(4): 355-359. |
| [15] | MAJAMAA K, SASAKI T, UITTO J. Inhibition of prolyl hydro-xylation during collagen biosynthesis in human skin fibroblast cultures by ethyl 3, 4-dihydroxybenzoate[J]. J Invest Dermatol, 1987, 89(4): 405-409. DOI:10.1111/1523-1747.ep12471775 |
| [16] | NAKAYAMA T, YAMADA M, OSAWA T, et al. Inhibitory effects of caffeic acid ethyl ester on H2O2-induced cytotoxicity and DNA single-strand breaks in Chinese hamster V79 cells[J]. Biosci Biotechnol Biochem, 1996, 60(2): 316-318. DOI:10.1271/bbb.60.316 |
| [17] |
ZHANG B Z. Studies on the allelopathy of Elodea nuttallii on Microcystis aeruginosa[D]. Wuhan: Institute of Hydrobiology, Chinese Academy of Sciences, 2007: 1-134.
张兵之.伊乐藻对铜绿微囊藻的化感作用研究[D].武汉: 中国科学院水生生物研究所, 2007: 1-134. http://www.wanfangdata.com.cn/details/detail.do?_type=degree&id=Y1616880 |
| [18] | DUARTE N, RAMALHETE C, VARGA A, et al. Multidrug resistance modulation and apoptosis induction of cancer cells by terpenic com-pounds isolated from Euphorbia species[J]. Anticancer Res, 2009, 29(11): 4467-4472. |
| [19] | REN C Q, YUAN W, DAI X Z, et al. Determination of β-sitosterol content in different species of herba Moghaniae by RP-HPLC-ELSD[J]. Med Plant, 2013, 4(7): 9-11. |
| [20] |
ZHOU Z Y, LU Q, LIU Y, et al. Research and development progress of stigmasterol[J].
Chin Modern Med, 2015, 22(24): 15-17. 周志远, 卢群, 刘洋, 等. 豆甾醇的研究及开发进展[J]. 中国当代医药, 2015, 22(24): 15-17. |
 2018, Vol. 26
2018, Vol. 26


