2. 中国科学院昆明植物研究所, 植物化学与西部植物资源国家重点实验室, 昆明 650204
2. State Key Laboratory of Phyto-chemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China
茶褐牛肝菌(Neoboletus brunneissimus Chiu)又名羊肝菌、黑羊肝、黑牛肝等, 是牛肝菌科(Boletaceae)牛肝菌属高等真菌[1-2],目前主要分布于云南、贵州及四川等中国西南地区,具有抗肿瘤、降血糖、保肝利胆、提高人体免疫力、调节内分泌等功能,有很高的食用价值和药用功效[3-4]。茶褐牛肝菌资源丰富,尚未有相关化学成分的报道,依靠云南资源优势,对该真菌进行了系统的化学成分研究,为高等真菌资源的充分利用提供理论依据, 同时也为完善牛肝菌科真菌的系统分类学提供化学成分方面的借鉴作用。利用现代分离纯化手段, 从其浸提液中提取分离得到18个化合物,其中化合物6、9、13和17为首次从牛肝菌科高等真菌中分离得到。
1 材料和方法 1.1 材料茶褐牛肝菌子实体于2016年6月购于昆明野生菌市场,真菌标本由中国科学院昆明植物研究所杨祝良教授鉴定为茶褐牛肝菌(Neoboletus brunneissimus Chiu),样品标本(编号KM20160601)现保存于昆明理工大学生命科学与技术学院药物分析课题组。
C18反相硅胶(日本YMC公司); D101大孔吸附树脂(天津兴南允能公司);葡聚糖凝胶Sephadex LH-20 (GE Helthcare公司);色谱纯乙腈、甲醇(CINC公司);柱色谱和薄层色谱用硅胶(青岛海洋化工);显色剂:5%~10%硫酸乙醇溶液,喷洒后给予适当加热。
1.2 仪器Bruker AM-400、AV Ⅲ-500和Ascend TM-600超导核磁共振波谱仪;Agilent 1100、1200系列高效液相色谱仪;Waters X select CSH C18色谱柱(19 mm× 150 mm, 5 μm);Waters X select HSS T3色谱柱(10 mm×150 mm, 5 μm);API-QSTAR-TOF超高压液相色谱三重四级杆串联质谱仪。
1.3 提取和分离新鲜茶褐牛肝菌子实体10 kg,切碎,用95%甲醇室温冷浸提取3次,每次72 h。合并提取液减压回收溶剂,得总浸膏300 g,将总浸膏用蒸馏水溶解,然后依次用石油醚、乙酸乙酯及正丁醇各萃取3次,得石油醚部分(35 g)、乙酸乙酯部分(20 g)和正丁醇部分(108 g)。
乙酸乙酯部分为黄色油状物,通过TLC展板显色对比发现,该部分极性跨度较大。称取60~80目硅胶50 g拌样,用200~300目硅胶200 g装柱,干法上样,以石油醚-丙酮(100:0~0:100,V/V)梯度洗脱,通过TLC展板显色对比,合并为Fr.A~Fr.D共4个部分。Fr.B部分析出大量无色针状结晶,用Sephadex LH-20凝胶柱(三氯甲烷-甲醇1:1)进行细分划段,再经300~400目硅胶柱色谱分离纯化(三氯甲烷等度洗脱),合并主点部分后用HPLC纯化(Waters X select色谱柱),得到化合物1(10.4 mg, 92% MeOH:H2O,tR=22 min)、2(6.9 mg,92% MeOH:H2O,tR=25 min)。Fr.C部分同样析出大量无色针状结晶,通过TLC展板显色对比,发现针晶部分与化合物1、2极性相似,故采用相似方法细分后用HPLC纯化(Waters X select色谱柱),得化合物3(4.2 mg, 95% MeOH:H2O, tR=20 min)、4(3.4 mg, 95% MeOH:H2O,tR=23 min)。Fr.D部分用Sepha- dex LH-20凝胶柱、正相硅胶柱、反相ODS中压柱等反复细分,最后用HPLC纯化(Waters X select色谱柱),得到化合物5(10.3 mg,90% MeOH:H2O,tR=17 min)、6(5.9 mg, 90% MeOH:H2O, tR=19 min)和8(10.3 mg,92% MeOH:H2O,tR=19 min)。
正丁醇部分用蒸馏水溶解后,用D101大孔吸附树脂柱对其进行粗分划段,以10%、30%、60%、90%甲醇水洗脱,划分为Fr.A~Fr.D共4个部分。用ODS中压柱对Fr.D部分进行细分划段,以50%、70%、90%甲醇水洗脱,划分为Fr.C1~Fr.C3共3个部分。运用Sephadex LH-20凝胶柱、正相硅胶柱、反相C18常压柱等对Fr.C1~Fr.C3部分进行反复细化除杂后,最后用HPLC纯化。用Acchrom X Amide色谱柱从Fr.C1部分得到化合物10(2.0 mg, 90% CH3CN: H2O,0.2%甲酸,tR=11 min)、11(10.2 mg, 92% CH3CN:H2O, 0.2%甲酸, tR=15 min)、12(102.5 mg, 88% CH3CN:H2O, 0.2%甲酸, tR=10 min)、16 (3.0 mg, 85% CH3CN:H2O,tR=11 min);用Acchrom X Amide色谱柱从Fr.C2部分得到化合物14(10.0 mg, 90% CH3CN:H2O, 0.2%甲酸, tR=12 min)、15(5.6 mg, 95% CH3CN:H2O,tR=25 min)。用Waters X select C18色谱柱从Fr.C3部分得到化合物7(59.5 mg,83% MeOH:H2O,tR=13 min)、9(3.6 mg,65% MeOH:H2O,tR=12 min)、13(4.0 mg,55% MeOH:H2O,tR=16 min)。最后用正相硅胶柱从Fr.C3部分得到化合物17(30.7 mg)和18(3.2 g)。
1.4 结构鉴定化合物1 无色针状结晶(氯仿); ESI-MSm/z: 429 [M + H]+; 1H NMR (500 MHz, CDCl3):δH 6.46 (1H, d, J = 8.4 Hz, H-7), 6.20 (1H, d, J = 8.4 Hz, H-6), 5.14 (1H, dd, J = 15.4, 7.2 Hz, H-23), 5.12 (1H, dd, J = 15.4, 7.2 Hz, H-22), 3.90 (1H, m, H-3), 2.08~1.49 (20H, m), 1.20 (3H, d, J= 6.4 Hz, H-21), 0.94 (3H, d, J = 6.6 Hz, H-28), 0.88 (3H, s, H-19), 0.86 (3H, d, J = 6.6 Hz, H-26), 0.84 (3H, s, H-18), 0.80 (3H, d, J = 6.6 Hz, H-27); 13C NMR (125 MHz, CDCl3): δC 34.6 (C-1), 29.8 (C-2), 66.3 (C-3), 36.8 (C-4), 82.2 (C-5), 135.4 (C-6), 130.6 (C-7), 79.4 (C-8), 51.0 (C-9), 36.7 (C-10), 23.3 (C-11), 39.2 (C-12), 44.5(C-13), 51.6 (C-14), 20.5 (C-15), 28.6 (C-16), 56.1 (C-17), 12.7 (C-18), 18.1 (C-19), 39.7 (C-20), 20.7 (C-21), 135.1 (C-22), 132.2 (C-23), 42.7 (C-24), 32.9 (C-25), 19.5 (C-26), 19.8 (C-27), 17.5 (C-28)。以上数据与文献[5]报道一致,故鉴定为5α, 8α-环二氧-(22E, 24R)-麦角甾-6, 22-二烯-3β-醇(5α, 8α-epidoxy-(22E, 24R)-ergosta-6, 22- dien-3β-ol)。
化合物2 无色针状结晶(氯仿); ESI-MS m/z: 427 [M + H]+; 1H NMR (600 MHz, CDCl3): δH 6.59 (1H, d, J = 8.2 Hz, H-7), 6.28 (1H, d, J = 8.2 Hz, H-6), 5.42 (1H, d, J = 4.2 Hz, H-11), 5.25 (1H, dd, J = 15.2, 7.6 Hz, H-23), 5.16 (1H, dd, J = 15.2, 7.6 Hz, H-22), 4.00 (1H, m, H-3), 2.08~1.49 (20H, m), 1.03 (3H, s, H-19), 1.00 (3H, d, J = 6.4 Hz, H-21), 0.99 (3H, d, J = 6.8 Hz, H-28), 0.91 (3H, d, J = 5.4 Hz, H-26), 0.84 (3H, d, J = 6.5 Hz, H-27), 0.81 (3H, s, H-18); 13C NMR (150 MHz, CDCl3): δC 32.5 (C-1), 30.6 (C-2), 66.3 (C-3), 36.0 (C-4), 82.7 (C-5), 135.4 (C-6), 130.7 (C-7), 78.3 (C-8), 142.5 (C-9), 37.9 (C-10), 119.7 (C-11), 41.1 (C-12), 43.6 (C-13), 48.1 (C-14), 21.3 (C-15), 28.6 (C-16), 55.8 (C-17), 12.9 (C-18), 25.5 (C-19), 39.9 (C-20), 20.7 (C-21), 135.1 (C-22), 132.4 (C-23), 42.7 (C-24), 33.0 (C-25), 19.9 (C-26), 19.6 (C-27), 17.5 (C-28)。以上数据与文献[6]报道一致, 故鉴定为5α, 8α-环二氧-(22E, 24R)-麦角甾-6, 9(11), 22-三烯-3β-醇[5α, 8α-epidioxy-(22E, 24R)-ergosta-6, 9(11), 22-trien-3β-ol]。
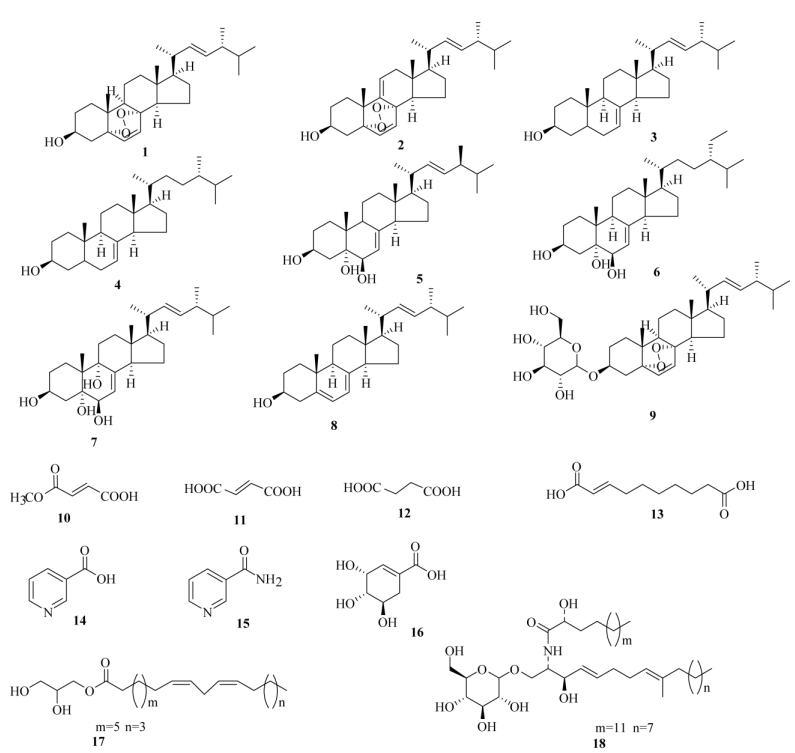
|
图 1 化合物1~18的结构 Fig. 1 Structure of compounds 1-18 |
化合物3 无色针状结晶(氯仿); ESI-MS m/z: 399 [M + H]+; 1H NMR (500 MHz, CDCl3): δH 6.16 (1H, m, H-7), 5.16 (2H, m, H-22, 23), 3.58 (1H, m, H-3), 1.00 (3H, d, J= 6.5 Hz, H-21), 0.92 (3H, d, J = 6.2 Hz, H-28), 0.84 (3H, d, J = 6.6 Hz, H-27), 0.79 (1H, d, J = 6.8 Hz, H-26), 0.78 (3H, s, H-19), 0.54 (3H, s, H-18); 13C NMR (125 MHz, CDCl3): δC 37.1 (C-1), 31.5 (C-2), 71.7 (C-3), 38.0 (C-4), 40.3 (C-5), 29.6 (C-6), 117.5 (C-7), 139.6 (C-8), 49.4 (C-9), 34.2 (C-10), 21.6 (C-11), 39.5 (C-12), 43.3 (C-13), 55.1 (C-14), 22.9 (C-15), 28.1 (C-16), 56.0 (C-17), 12.1 (C-18), 13.1 (C-19), 40.5 (C-20), 21.1 (C-21), 135.7 (C-22), 131.2 (C-23), 42.8 (C-24), 33.1 (C-25), 20.0 (C-26), 19.7 (C-27), 17.6 (C-28)。以上数据与文献[7]报道一致,故鉴定为(22E, 24R)-麦角甾-7, 22-二烯- 3β-醇[(22E, 24R)-ergosta-7, 22-dien-3β-ol]。
化合物4 无色针状结晶(氯仿); ESI-MS m/z: 401 [M + H]+; 1H NMR (500 MHz, CDCl3): δH 6.16 (1H, m, H-7), 3.61 (1H, m, H-3), 0.93 (d, J= 6.2 Hz, H-21), 0.86 (1H, d, J = 6.6 Hz, H-21), 0.84 (3H, s, H-19), 0.79 (3H, d, J = 6.8 Hz, H-27), 0.78 (6H, d, J = 6.6 Hz, H-26, 28), 0.53 (s, H-18); 13C NMR (125 MHz, CDCl3):δC 37.2 (C-1), 31.5 (C-2), 71.1 (C-3), 38.0 (C-4), 39.6 (C-5), 29.7 (C-6), 117.4 (C-7), 139.6 (C-8), 49.5 (C-9), 34.2 (C-10), 21.6 (C-11), 39.1 (C-12), 43.4 (C-13), 55.0 (C-14), 23.0 (C-15), 28.1 (C-16), 56.0 (C-17), 11.9 (C-18), 13.0 (C-19), 36.6 (C-20), 19.0 (C-21), 33.7 (C-22), 30.7 (C-23), 40.0 (C-24), 31.5 (C-25), 17.6 (C-26), 20.5 (C-27), 15.4 (C-28)。以上数据与文献[7]报道一致,故鉴定为(24S)-麦角甾-7-烯- 3β-醇[(24S)-ergosta-7-en-3β-ol]。
化合物5 无色针状结晶(氯仿); ESI-MS m/z: 431 [M + H]+; 1H NMR (500 MHz, CDCl3): δH 5.25 (1H, dd, J = 15.2, 7.6 Hz, H-23), 5.24 (1H, dd, J = 15.2, 7.6 Hz, H-22), 5.17 (1H, dd, J = 4.7, 2.5 Hz, H- 7), 4.22 (1H, brs, H-3), 4.02 (1H, m, H-6), 1.52 (3H, s, H-19), 1.02 (3H, d, J= 6.2 Hz, H-21), 0.98 (3H, d, J = 6.6 Hz, H-28), 0.96 (6H, d, J = 6.8 Hz, H-26, 27), 0.54 (3H, s, H-18); 13C NMR (125 MHz, CDCl3): δC 33.3 (C-1), 31.0 (C-2), 67.8 (C-3), 40.0 (C-4), 76.4 (C-5), 73.6 (C-6), 118.1 (C-7), 143.6 (C-8), 43.6 (C-9), 37.5 (C-10), 23.5 (C-11), 39.6 (C-12), 44.1 (C-13), 55.3 (C-14), 22.5 (C-15), 28.5 (C-16), 56.6 (C-17), 12.6 (C-18), 18.6 (C-19), 41.0 (C-20), 21.4 (C-21), 136.2 (C-22), 132.6 (C-23), 43.5 (C-24), 33.6 (C-25), 20.2 (C-26), 19.9 (C-27), 17.9 (C-28)。以上数据与文献[8]报道一致,故鉴定为(22E, 24R)-麦角甾-7, 22-二烯-3β, 5α, 6β-三醇[(22E, 24R)-ergosta-7, 22-dien-3β, 5α, 6β-triol]。
化合物6 无色针状结晶(氯仿); ESI-MSm/z: 489 [M + Na]+; 1H NMR (600 MHz, CDCl3): δH 5.44 (1H, brs, H-7), 4.15 (1H, m, H-3), 3.65 (1H, d, J = 5.4 Hz, H-6), 1.06 (3H, s, H-19), 0.95 (3H, d, J = 6.8 Hz, H-21), 0.86 (3H, t, J = 7.6 Hz, H-29), 0.85 (3H, d, J = 6.6 Hz, H-26), 0.83 (3H, d, J = 6.8 Hz, H-27), 0.56 (3H, s, H-18); 13C NMR (150 MHz, CDCl3): δC 33.7 (C-1), 31.6 (C-2), 67.5 (C-3), 39.2 (C-4), 76.0 (C-5), 74.1 (C-6), 120.4 (C-7), 141.5 (C-8), 43.6 (C-9), 36.9 (C-10), 23.4 (C-11), 37.9 (C-12), 43.7 (C-13), 55.0 (C-14), 22.3 (C-15), 29.9 (C-16), 56.2 (C-17), 12.2 (C-18), 17.6 (C-19), 39.9 (C-20), 20.6 (C-21), 32.5 (C-22), 31.0 (C-23), 41.9 (C-24), 33.8 (C-25), 19.1 (C-26), 18.7 (C-27), 28.0 (C-28), 15.5 (C-29)。以上数据与文献[9]报道一致,故鉴定为(24S)-乙基胆甾烯-7-烯-3β, 5α, 6β-三醇[(24S)-ethyl-cholest-7-ene-3β, 5α, 6β-triol]。
化合物7 白色粉末(甲醇); ESI-MS m/z: 447 [M + H]+; 1H NMR (500 MHz, CDCl3): δH 5.16 (1H, dd, J = 15.4, 7.4 Hz, H-22), 5.14 (1H, dd, J = 15.4, 7.4 Hz, H-23), 5.14 (1H, d, J = 3.3 Hz, H-7), 3.80 (1H, m, H-3), 3.46 (1H, d, J = 3.3 Hz, H-6), 2.29 (1H, m, H-14), 1.05 (3H, J= 6.6 Hz, H-21), 0.90 (3H, s, H-19), 0.82 (1H, d, J = 6.7 Hz, H-28), 0.81 (3H, d, J = 7.6 Hz, H-26), 0.80 (3H, d, J = 7.6 Hz, H-27), 0.61 (3H, s, H-18); 13C NMR (125 MHz, CDCl3):δC 27.2 (C-1), 30.6 (C-2), 67.3 (C-3), 40.5 (C-4), 75.1 (C-5), 72.9 (C-6), 119.8 (C-7), 143.3 (C-8), 77.8 (C-9), 40.8 (C-10), 28.5 (C-11), 35.5 (C-12), 44.0 (C-13), 51.0 (C-14), 23.2 (C-15), 28.3 (C-16), 56.2 (C-17), 12.0 (C-18), 21.7 (C-19), 40.0 (C-20), 21.2 (C-21), 135.8 (C-22), 132.4 (C-23), 43.2 (C-24), 33.4 (C-25), 20.1 (C-26), 19.8 (C-27), 17.8 (C-28)。以上数据与文献[10]报道一致,故鉴定为(22E, 24R)-麦角甾-7, 22-二烯-3β, 5α, 6β, 9α-四烯[(22E, 24R)-ergosta-7, 22-diene-3β, 5α, 6β, 9α-tetrol)。
化合物8 无色针状结晶(甲醇); ESI-MS m/z: 397 [M + H]+; 1 H NMR (500 MHz, CDCl3): δH 5.50 (1H, d, J = 5 Hz, H-6), 5.33 (1H, s, H-7), 5.32 (1H, dd, J = 15.2, 7.4 Hz, H-23), 5.15 (1H, dd, J = 15.2, 7.4 Hz, H-22), 3.52 (1H, m, H-3), 1.00 (2H, d, J = 12.5 Hz, H-4), 0.98 (3H, d, J = 6.6 Hz, H-21), 0.87 (3H, s, H-19), 0.8 (3H, d, J = 6.6 Hz, H-28), 0.76 (6H, d, J = 6.6 Hz, H-26, 27), 0.58 (3H, s, H-18); 13C NMR (125 MHz, CDCl3): δC 38.7 (C-1), 31.8 (C-2), 70.2 (C-3), 40.6 (C-4), 140.3 (C-5), 119.8 (C-6), 116.6 (C-7), 141.6 (C-8), 46.6 (C-9), 37.3 (C-10), 21.4 (C-11), 39.4 (C-12), 43.2 (C-13), 54.8 (C-14), 23.3 (C-15), 28.6 (C-16), 56.0 (C-17), 12.3 (C-18), 16.4 (C-19), 40.8 (C-20), 21.3 (C-21), 135.9 (C-22), 132.3 (C-23), 43.1(C-24), 33.4 (C-25), 20.1 (C-26), 19.8 (C-27), 17.8 (C-28)。以上数据与文献[11]报道一致,故鉴定为(22E, 24R)-麦角甾-5, 7, 2-三烯-3β-醇[(22E, 24R)-ergosta-5, 7, 22-trien-3β-ol]。
化合物9 白色无定型粉末(甲醇); ESI-MS m/z: 613 [M + Na]+; 1H NMR (500 MHz, CDCl3): δH 6.28 (1H, d, J = 8.4 Hz, H-6), 6.00 (1H, d, J = 8.4 Hz, H-7), 5.22 (1H, dd, J = 15.2, 7.4 Hz, H-22), 5.18 (1H, dd, J = 15.2, 7.4 Hz, H-23), 4.18 (2H, d, J = 8.6 Hz, H-6′), 1.16 (3H, d, J = 6.4 Hz, H-21), 0.98 (3H, d, J = 6.6 Hz, H-28), 0.96 (3H, s, H-19), 0.88 (3H, d, J = 6.6 Hz, H-27), 0.84 (3H, d, J = 6.6 Hz, H-26), 0.78 (3H, s, H-18); 13C NMR (125 MHz, CDCl3): δC 28.2 (C-1), 33.2 (C-2), 73.1 (C-3), 38.8 (C-4), 81.8 (C-5), 134.8 (C-6), 130.3 (C-7), 79.3 (C-8), 51.7 (C-9), 36.6 (C-10), 20.3 (C-11), 34.4 (C-12), 44.4 (C-13), 51.2 (C-14), 22.9 (C-15), 27.7 (C-16), 55.8 (C-17), 12.5 (C-18), 17.5 (C-19), 39.3 (C-20), 19.3 (C-21), 135.0 (C-22), 131.9 (C-23), 42.4 (C-24), 32.6 (C-25), 19.0 (C-26), 20.1 (C-27), 17.0 (C-28), 101.1 (C-1′), 73.8 (C-2′), 75.6 (C-3′), 69.7 (C-4′), 76.1 (C-5′), 61.2 (C- 6′)。以上数据与文献[12]报道一致,故鉴定为3β-O-吡喃葡萄糖基-5α, 8α-环二氧-(22E, 24R)-麦角-6, 22-二烯(3β-O-Glucopyranosyl-5α, 8α-epidioxiergosta-(22E, 24R)-ergosta-6, 22-diene)。
化合物10 白色粉末(甲醇); 1H NMR(500 MHz, CD3OD): δH 6.77 (2H, d, J = 15.5 Hz, H-2, 3), 3.80 (3H, s, OCH3); 13C NMR (125 MHz, CD3OD): δC 168.0 (C-1), 134.2 (C-2), 135.7 (C-3), 167.1 (C-4), 52.9 (C-5)。与文献[13]报道一致,故鉴定为富马酸单甲酯(monomethyl fumarate)。
化合物11 白色粉末(甲醇); 1H NMR(500 MHz, DMSO-d6): δH 13.07 (2H, brs, H-1, 4), 6.60 (2H, s, H-2, 3); 13C NMR (125 MHz, DMSO-d6): δC 166.1 (C-1, 4), 134.0 (C-2, 3)。以上数据与文献[14]报道一致,故鉴定为富马酸(fumaric acid)。
化合物12 白色粉末(甲醇); 1H NMR(500 MHz, CDCl3): δH 13.1(2H, s, COOH), 3.00 (4H, s, CH2); 13C NMR (125 MHz, CDCl3): δC 175.4 (C-1, 4), 30.1 (C-2, 3)。以上数据与文献[15]报道一致,故鉴定为琥珀酸(succinic acid)。
化合物13 无色油状(甲醇); ESI-MS m/z: 199 [M-H]−; 1H NMR (500 MHz, CD3OD):δH 6.94 (1H, m, H-3), 5.77 (1H, d, J = 15.4 Hz, H-2), 2.28 (2H, m, H-4), 2.22 (2H, m, H-9), 1.60 (2H, m, H-8), 1.48 (2H, m, H-7), 1.26 (4H, m, H-5, 6); 13C NMR (125 MHz, CD3OD): δC 170.1 (C-1), 122.5 (C-2), 151.2 (C-3), 33.1 (C-4), 29.9 (C-5, 6), 29.1 (C-7), 26.0 (C-8), 34.9 (C-9), 177.7 (C-10)。以上数据与文献[16]报道一致,故鉴定为反-2-癸烯二酸(trans-2-dece- nedioic acid)。
化合物14 白色粉末(甲醇); ESI-MS m/z: 124 [M + H]+; 1H NMR (500 MHz, CD3OD): δH 9.11 (1H, s, H-2), 8.71 (1H, d, J = 12.6 Hz H-6), 8.40 (1H, m, H-4), 7.55 (1H, m, H-5); 13C NMR (125 MHz, CD3OD): δC 151.2 (C-2), 128.8 (C-3), 139.2 (C-4), 125.2 (C-5), 153.6 (C-6), 167.9 (C-7)。以上数据与文献[17]报道一致,故鉴定为烟酸(nicotinic acid)。
化合物15 白色粉末(甲醇); ESI-MS m/z: 123 [M + H]+; 1H NMR (500 MHz, CD3OD): δH 9.02 (1H, s, H-2), 8.66 (1H, dd, J = 4.9, 1.6 Hz H-6), 8.26 (1H, m, H-4), 7.52 (1H, dd, J = 4.8, 4.8 Hz, H-5); 13C NMR (125 MHz, CD3OD):δC 149.5 (C-2), 131.4 (C- 3), 137.3 (C-4), 125.1 (C-5), 152.8 (C-6), 169.8 (C- 7)。以上数据与文献[18]报道一致,故鉴定为烟酰胺(nicotinamide)。
化合物16 白色粉末(甲醇); 1H NMR (600 MHz, CD3OD): δH 6.78 (1H, s, H-2), 4.33 (1H, s, H-3), 4.00 (1H, dd, J = 12.2, 5.4 Hz, H-4), 3.67 (1H, dd, J = 7.2, 4.3 Hz, H-5), 2.71 (1H, dd, J= 17.9, 4.3 Hz, H-6a), 2.22 (1H, dd, J = 17.9, 4.3 Hz, H-6b); 13C NMR (150 MHz, CD3OD): δC 131.5 (C-1), 138.5 (C- 2), 72.7 (C-3), 68.6 (C-4), 67.5 (C-5), 31.8 (C-6), 170.6 (COOH)。以上数据与文献[19]报道一致,故鉴定为莽草酸(shikimic acid)。
化合物17 无色油状物(氯仿); EI-MS m/z: 355 [M + H]+; 1H NMR (500 MHz, CDCl3): δH 5.20 (4H, m, H-9, 10, 12, 13), 3.99 (1H, d, J = 2.2 Hz, H- 1′a), 3.95 (1H, d, J = 2.3 Hz, H-1′b), 3.74 (1H, dd, J = 5.6, 3.8 Hz, H-2′), 3.51 (1H, dd, J = 11.5, 3.8 Hz, H- 3′b), 3.42 (1H, dd, J = 11.6, 6.0 Hz, H-3′a), 2.55 (2H, t, J = 5.6 Hz, H-11), 2.15 (2H, t, J= 7.6 Hz, H-2), 1.90~1.12 (20H, m, H-3~8, H-14~17), 0.68 (3H, t, J = 6.8 Hz, H-18); 13C NMR (125 MHz, CDCl3): δC 173.4 (C-1), 34.0 (C-2), 31.2 (C-3), 29.6~29.0 (C-4~ C-8), 129.7 (C-9), 129.2 (C-10), 28.8 (C-11), 127.5 (C-12), 127.0 (C-13), 26.7~22.0 (C-14~C-17), 13.8 (C-18), 64.5 (C-1′), 69.7 (C-2′), 62.9 (C-3′)。以上数据与文献[20]报道一致,故鉴定为亚油酸-α-甘油酯(linoleic acid-α-glyceride)。
化合物18 白色粉末(甲醇); ESI-MS m/z: 726 [M-H]−; 1H NMR (500 MHz, CD3OD):δH 7.15 (1H, d, J = 8.7 Hz, NH), 5.72 (1H, m, H-5), 5.50 (1H, m, H-4), 5.48 (1H, m, H-8), 5.14 (1H, d, J = 7.6 Hz, H-1〞), 4.24 (1H, m, H-3), 4.13 (1H, m, H-2), 4.10 (1H, m, H-2′), 3.98 (1H, dd, J = 10.7, 5.4 Hz, H-1b), 3.97 (1H, brs, H-6a〞), 3.87 (1H, dd, J = 11.8, 5.6 Hz, H- 6b〞), 3.68 (1H, m, H-1a), 3.66 (1H, m, H-4〞), 3.64 (1H, m, H-3〞), 3.33 (1H, m, H-2〞), 3.17 (1H, m, H-5〞), 2.14 (4H, m, H-6, 7) 1.95 (2H, m, H-10), 1.41 (3H, s, H-19), 1.40 (2H, m, H-3′), 1.39 (2H, m, H-11), 1.28 (30H, brs, H-4′~H-13′, H-15′, H-12-15), 0.90 (3H, t, J = 6.9 Hz, H-18), 0.77 (3H, t, J = 6.8 Hz, H-16′); 13C NMR (125 MHz, CD3OD): δC 69.8 (C-1), 54.6 (C-2), 73.1 (C-3), 134.7 (C-4), 131.1 (C-5), 33.8 (C-6), 29.2 (C-7), 124.8 (C-8), 136.7 (C-9), 40.8 (C-10), 30.9~30.7 (C-11~C-15), 33.1 (C-16), 28.7 (C-17), 14.5 (C-18), 23.8 (C-19), 177.3 (C-1′), 72.9 (C-2′), 35.9 (C-3′), 30.7~30.9 (C-4′~C-13′), 30.6 (C- 14′), 26.2 (C-15′), 16.2 (C-16′), 104.7 (C-1〞), 75.0 (C- 2〞), 77.8 (C-3〞), 71.5 (C-4〞), 78.0 (C-5〞), 62.6 (C- 6〞)。以上数据与文献[21-22]报道一致,故鉴定为1-O-吡喃葡萄糖基-(2S, 3R, 4E, 8E, 2′R)-2-N-(2′-羟基棕榈酰)-9-甲基-4, 8-鞘氨醇(1-O-β-D-glucopyranosyl- (2S, 3R, 4E, 8E, 2′R)-2-N-(2′-hydroxypalmitoyl)-9-methyl-4, 8-sphingadienine)。
2 结果和讨论本研究通过现代的分离手段和技术,从茶褐牛肝菌子实体中分离鉴定了18个化合物,分别为5α, 8α-环二氧-(22E, 24R)-麦角甾-6, 22-二烯-3β-醇(1)、5α, 8α-环二氧-(22E, 24R)-麦角甾-6, 9(11), 22-三烯-3β-醇(2)、(22E, 24R)-麦角甾-7, 22-二烯-3β-醇(3)、(24S)-麦角甾-7-烯-3β-醇(4)、(22E, 24R)-麦角甾-7, 22-二烯-3β, 5α, 6β-三醇(5)、(24S)-乙基胆甾烯-7-烯- 3β, 5α, 6β-三醇(6)、(22E, 24R)-麦角甾-7, 22-二烯-3β, 5α, 6β, 9α-四烯(7)、(22E, 24R)-麦角甾-5, 7, 2-三烯-3β-醇(8)、3β-O-吡喃葡萄糖基-5α, 8α-环二氧-(22E, 24R)-麦角-6, 22-二烯(9)、富马酸单甲酯(10)、富马酸(11)、琥珀酸(12)、反-2-癸烯二酸(13)、烟酸(14)、烟酰胺(15)、莽草酸(16)、亚油酸-α-甘油酯(17)和脑苷脂(18),所有化合物均为首次从茶褐牛肝菌中发现。据报道麦角甾醇的过氧化物和富马酸单甲酯有抑菌活性[23-26],麦角甾醇有抗肿瘤,抗氧化活性[25-26],莽草酸对肝癌细胞HepG2增殖具有抑制作用[27],烟酰胺药理活性丰富,具有抗氧化、抗炎、代谢调控作用,相关信号调控通路等药理作用[28]。这些化合物的活性与野生牛肝菌具有抗肿瘤、保肝利胆、提高人体免疫力、调节内分泌等功能一致[3],为茶褐牛肝菌的开发利用提供科学依据,同时也丰富了茶褐牛肝菌的化合物库。
| [1] | WU G, ZHAO K, LI Y C, et al. Four new genera of the fungal family Boletaceae[J]. Fungal Div, 2016, 81(1): 1-24. DOI:10.1007/s13225-015-0322-0 |
| [2] |
DAI Y C, ZHOU L W, YANG Z L, et al. A revised checklist of edible fungi in China[J].
Mycosystema, 2010, 29(1): 1-21. 戴玉成, 周丽伟, 杨祝良, 等. 中国食用菌名录[J]. 菌物学报, 2010, 29(1): 1-21. DOI:10.13346/j.mycosystema.2010.01.022 |
| [3] |
LIU J, GAO M, WU K F, et al. Study on health function of natural Boletus[J].
Chin J Public Health Eng, 2011, 10(3): 224-226. 刘佳, 高敏, 吴克枫, 等. 野生牛肝菌保健功能研究[J]. 中国卫生工程学, 2011, 10(3): 224-226. |
| [4] | WASSER S P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms[J]. Appl Microbiol Biotechnol, 2011, 89(5): 1323-1332. DOI:10.1007/s00253-010-3067-4 |
| [5] |
Ma B J, Shen J W, Yu H Y, et al. Chemical composition of the fruiting bodies of Helvella elastic[J].
Acta Bot Boreali-Occid Sin, 2009, 29(10): 2115-2117. 麻兵继, 申进文, 余海尤, 等. 马鞍菌子实体化学成分研究[J]. 西北植物学报, 2009, 29(10): 2115-2117. |
| [6] | KIM K H, CHOI S U, PARK K M, et al. Cytotoxic constituents of Amanita subjunquillea[J]. Arch Pharmacal Res, 2008, 31(5): 579-586. DOI:10.1007/s12272-001-1196-3 |
| [7] |
GAO J M, SHEN J, YANG X, et al. The constituents of Russula ochroleuca basidiomycetes[J].
Acta Bot Yunnan, 2001, 23(3): 385-393. 高锦明, 沈杰, 杨雪, 等. 黄白红菇的化学成分[J]. 云南植物研究, 2001, 23(3): 385-393. DOI:10.3969/j.issn.2095-0845.2001.03.019 |
| [8] | LI H J, LUO Y G, HE Z H, et al. Phytochemical study on Zehneria maysorensis[J]. Nat Prod Res Dev, 2006, 18(3): 411-414. DOI:10.16333/j.1001-6880.2006.03.014 |
| [9] | PICCIALLI V, SICA D. Four new trihydroxylated sterols from the sponge Spongionella gracilis[J]. J Nat Prod, 1987, 50(5): 915-920. DOI:10.1021/np50053a024 |
| [10] |
E H C, ZHOU W, LIU B S, et al. Secondary metabolites from the fungus Engyodontium album associated with the South China Sea starfish Anthenea pentagonula[J].
Chin J Mar Drug, 2013, 32(6): 8-12. 鄂恒超, 周巍, 刘宝姝, 等. 中国南海真五角海星共附生白色侧齿霉菌的次生代谢产物研究[J]. 中国海洋药物, 2013, 32(6): 8-12. DOI:10.13400/j.cnki.cjmd.2013.06.005 |
| [11] |
GONG J, TANG H, LIU B S, et al. Steroids from fungus Engyodon-tium album associated with the South China Sea cucumber Holothuria nobilis Selenka[J].
Acad J Second Mil Med Univ, 2013, 34(3): 310-314. 宫俊, 汤华, 刘宝姝, 等. 中国南海黑乳海参共附生白色侧齿霉菌中的甾体类成分[J]. 第二军医大学学报, 2013, 34(3): 310-314. DOI:10.3724/SP.J.1008.2013.00310 |
| [12] | LI X J, GAO J M, CHEN H, et al. Toxins from a symbiotic fungus, Leptographium qinlingensis, associated with Dendroctonus armandi, and their in vitro toxicities to Pinus armandi seedlings[J]. Eur J Plant Pathol, 2012, 134(2): 239-247. DOI:10.1007/s10658-012-9981-9 |
| [13] |
ZHANG Y, RUAN H L, ZHANG Y H, et al. Studies on chemical constituents of Cirseum henryi in western Hubei[J].
Herald Med, 2007, 26(12): 1425-1426. 张悦, 阮汉利, 张勇慧, 等. 鄂西大蓟化学成分的研究[J]. 医药导报, 2007, 26(12): 1425-1426. DOI:10.3870/j.issn.1004-0781.2007.12.012 |
| [14] | ZHNG H L, ZHANG Q W, ZHANG X Q, et al. Chemical constituents from the roots of Morinda officinalis[J]. Chin J Nat Med, 2010, 8(3): 192-195. DOI:10.3724/SP.J.1009.2010.00192 |
| [15] |
MA B J, LIU J K. Chemical study on Russula densifolia[J].
Nat Prod Res Dev, 2005, 17(1): 29-32. 麻兵继, 刘吉开. 密褶红菇化学成分研究[J]. 天然产物研究与开发, 2005, 17(1): 29-32. DOI:10.16333/j.1001-6880.2005.01.008 |
| [16] | KWOK O C H, PLATTNER R, WEISLEDER D, et al. A nematicidal toxin from Pleurotus ostreatus NRRL 3526[J]. J Chem Ecol, 1992, 18(2): 127-136. DOI:10.1007/BF00993748 |
| [17] |
TANG L C, WANG N, YAO H P, et al. Chemical components of the Hevea brasiliensis skim[J].
Chem Ind For Prod, 2013, 33(1): 125-129. 汤丽昌, 王宁, 姚海萍, 等. 天然橡胶胶清化学成分的研究[J]. 林产化学与工业, 2013, 33(1): 125-129. DOI:10.3969/j.issn.0253-2417.2013.01.024 |
| [18] |
SHI B J, CHOU G X, WANG Z T. Chemical constituents from Senecio nemorensis[J].
J Chin Pharm Univ, 2010, 41(1): 26-28. 石宝俊, 侴桂新, 王峥涛. 林荫千里光的化学成分[J]. 中国药科大学学报, 2010, 41(1): 26-28. DOI:10.11665/j.issn.1000-5048.20100104 |
| [19] | ZHANG J M, SHI X F, MA Q H, et al. Chemical constituents from pine needles of Cedrus deodara[J]. Chem Nat Compd, 2011, 47(2): 272-274. DOI:10.1007/s10600-011-9901-9 |
| [20] | MURATA T, MORI N, NISHIDA R. Larval feeding stimulants for a rutaceae-feeding swallowtail butterfly, Papilio xuthus L. in Citrus unshiu leaves[J]. J Chem Ecol, 2011, 37(10): 1099-1109. DOI:10.1007/s10886-011-0022-5 |
| [21] |
YANG W Q. Total synthesis of pulverolide and study on chemical constituents of seven macrofungi[D]. Kunming: Kunming Institute of Botany, Chinese Academy of Sciences, 2010: 1-191.
杨婉秋. 七种高等真菌化学成分研究和Pulverolide全合成研究[D]. 昆明: 中国科学院昆明植物研究所, 2010: 1-191. |
| [22] |
HU L, DING Z H, LIU J K. The chemical constituents of Basidio-mycetes Boletopsis grisea[J].
Acta Bot Yunnan, 2002, 24(5): 667-670. 胡琳, 丁智慧, 刘吉开. 灰黑拟牛肝菌的化学成分[J]. 云南植物研究, 2002, 24(5): 667-670. DOI:10.3969/j.issn.2095-0845.2002.05.015 |
| [23] |
MA B J, WEN C N, WU T T, et al. Study on the anti-bacterial activity of ergosterol peroxide[J].
Food Res Dev, 2012, 33(7): 42-44. 麻兵继, 文春南, 吴婷婷, 等. 麦角甾醇过氧化物的抑菌活性研究[J]. 食品研究与开发, 2012, 33(7): 42-44. |
| [24] |
LU W P, LIU C C. Synthesis and mould inhibiting activities of new aseptic monomethyl fumarate[J].
Food Res Dev, 2006, 27(10): 51-53. 卢卫平, 刘长春. 高效防霉剂富马酸单甲酯的合成与抑菌活性研究[J]. 食品研究与开发, 2006, 27(10): 51-53. DOI:10.3969/j.issn.1005-6521.2006.10.016 |
| [25] |
GAO H, SHI D F, YANG D, et al. Antitumor actvity of Ergosterol from Agaricus blazei Murrill and its mechanism[J].
Edible Fungi China, 2011, 30(6): 35-39. 高虹, 史德芳, 杨德, 等. 巴西菇麦角甾醇抗肿瘤活性及作用机理初探[J]. 中国食用菌, 2011, 30(6): 35-39. DOI:10.13629/j.cnki.53-1054.2011.06.016 |
| [26] |
QIN W G G, KONG Y S, JIANG W, et al. Isolation and identification of main substance ergosterol from the endophytic fungus MG-9 and its antioxidant activity analysis[J].
J Chin Three Gorges Univ (Nat Sci), 2017, 39(2): 108-112. 秦王阁阁, 孔玉珊, 蒋维, 等. 内生真菌MG-9的主物质麦角甾醇的分离鉴定及抗氧化活性分析[J]. 三峡大学学报(自然科学版), 2017, 39(2): 108-112. DOI:10.13393/j.cnki.issn.1672-948X.2017.02.023 |
| [27] |
GE Z T, WU L, ZHANG W M. The research of effects of shikimic acid on BLM helicase activities and hepatoma cells[J].
Chin Forei Med Treat, 2016, 35(16): 9-11. 葛章文, 吴利, 张望明. 莽草酸抑制BLM解旋酶活性及肝癌细胞的研究[J]. 中外医疗, 2016, 35(16): 9-11. DOI:10.16662/j.cnki.1674-0742.2015.16.009 |
| [28] |
YANG C, ZHEN Y Q, DAI M. Advances in pharmacological effects of nicotinamide[J].
J Clin Pulmon Med, 2011, 16(12): 1914-1916. 杨驰, 郑咏秋, 戴敏. 烟酰胺药理作用研究进展[J]. 临床肺科杂志, 2011, 16(12): 1914-1916. DOI:10.3969/j.issn.1009-6663.2011.12.044 |
 2018, Vol. 26
2018, Vol. 26


