2. 南京林业大学竹类研究所, 南京 210037
2. Bamboo Research Institute of Nanjing Forestry University, Nanjing 210037, China
Most bamboos (Bambusoideae) are perennial woody plants and they are divided into approximately 1 400 species growing mainly in Asia[1]. Flowering is an important phase in plant's life cycle. Studies on bamboo embryology are very hysteretic compared to other angiosperms. The researches on bamboo embryology were carried out currently only in Chimo- nobambusa marmorea[2], Drepanostachyum micro- phyllum[3], Dendrocalamus sinicus[4], Phyllostachys praecox[5], Menstruocalamus sichuanensis[6], Shibataea chinensis[7-8], Arundinariasimonii f. heterophylla[7, 9], which were caused by the long reproductive cycle of bamboo species.
In recent years, Bambusa multiplex in Jiangsu Province bloomed in succession. Bambusa multiplex, a bamboo species with good ornamental value, is belonged to Bambusa, Poaceae. The cultivated area of B. multiplex is very wide in the southeast and southwest of China because of high economic value in China. Usually it is used as hedges due to its beautiful posture. It is also one of the sympodial bamboo species with cold resistance and can live safely through the winter in the northern margin in subtropical zone.
The previous researches of Bambusa multiplex focused on the genetic diversity, rapid propagation and lignin synthesis genes, resistance, and so on[10-14]. In recent years, Bambusa multiplex in Jiangsu Province bloomed in succession with low setting rate. This provides not only materials to study flowering biological characteristics of B. multiplex, but also a unique opportunity for embryology research. The microscopic characteristics of megaspore and female gametophyte development of B. multiplex have not been systematically studied before. Abnormal sexual development is one of the main reasons of low setting rate in plants[15-16], and researches on bamboo flowering mechanism have a slow progress[17]. Therefore in this paper, we described the anatomic characteristics of megaspore and female gametophyte on B. multiplex using the method of scanning electron microscopy and the traditional paraffin section in order to explore whether female reproductive and developmental abnormalities is related to its low setting rate.
As consequence, the aims of this study are to increase our embryological knowledge of Bambusa multiplex, and to compare the embryological characters of B. multiplex to other bamboos in Bambusoideae. These would supply information on the reproductive biology of this species, and will contribute important knowledge concerning embryo- logical data on bamboo plants.
1 Materials and methods 1.1 MaterialsFlower buds, spikelet or florets of Bambusa multiplex were collected from Nanjing Forestry University at AM 8:00-10:00 every 3 days from March to June, and daily from middle April and early May in recent years. The inflorescence or florets at different development stage were collected and fixed with 70% FAA [formalin (38% formaldehyde) 5 mL; glacial acetic acid 5 mL; 70% alcohol 90 mL] at first. Before paraffin section, each flower was dissected, measured and recorded the length of anthers by vernier caliper.
1.2 MethodsFlower buds were fixed with 70% FAA at 5℃. At first, the samples were washed in 95% ethanol and 100% butanol successively (2×2-3 h), and then the samples were kept for 2-3 h in solution of xylol and butanol in proportions 1:3, 1:1 and 3:1 succe- ssively, and then treated with pure xylol for 2 h (3×), placed in a mixture of xylol-paraffin and kept at 58℃, stored overnight allowing the xylol to evaporate. Then the samples were kept in pure paraffin for 2-3 h (3×). Finally, the samples were imbedded in paraffin.
The samples were treated with Ehrfich's haema- toxylin as a whole, and flaked them by the conven- tional paraffin method at a thickness of 8 μm using a microtome Leica RM2255. Some samples were triple stained by hematoxylin, sarranine and fast green. The images were captured with a microscope Leica DM5000B[18]. At least 3 replications were carried out for each experiment and micrograghed.
For scanning electron microscopy (SEM), the samples were fixed with 70% FAA, then dehydrated through an ethanol series. Thereafter, they were critical- point-dried[19]. The samples were then mounted onto copper sheet and coated with gold. Observations and micrograghs were performed with a FEI-QUANTA- 200 scanning electron microscope.
2 Results and analysis 2.1 Pistil morphology and structureThe mature pistil of Bambusa multiplex consists of three stigmas, one short style, and one ovary. During pistil primordial period, firstly the top differentiates into 3 small protrusions called stigma primordium, then developes into 3-lobed stigma (Fig. 1: A, B), young stigmatic branches are short (Fig. 1: C, D). When ovary matures, stigmas have more branches (Fig. 1: E). The oblong expanded ovary is 1 mm in length and contains an anatropous, bitegmic and tenuinucellate ovule. There are many short furs around the upper part of ovary (Fig. 1: F).

|
Fig. 1 Gynoecium of Bambusa multiplex under SEM. A-B: Stigma primordium, the top differentiated into 3 small protrusions, then developed into 3-lobed stigma; C-D: Young short feather-like branches of stigma; E: More and more branches along the growth of pistil stigma; F: When ovary matures, there are a lot of short furs around the upper of ovary. |
At the early period of ovary development for Bambusa multiplex, a mass of cells at placenta initiated under the epidermis of young carpels, which are called the ovule primordia. When stamens (anthers) are 0.5-1.5 mm in length, ovule primordia begins to differentiate. Archesporial cell with thick cytoplasm and large cell nuclei under the epidermis is larger than the other cells. The ovule is tenuinucellate (Fig. 2: A). Archesporial cell continues to extend longitudinally, the volume is increasing, and cell nucleus gets larger, then archesporial cell develops directly into the mega- sporocytes (Fig. 2: B). Later, the megaspore mother cell undergoes meiosis and divides to form a linear megaspore tetrad (Fig. 2: C, D), and the one near micropyle end develops into functional megaspore (Fig. 2: E), while the others degenerate.
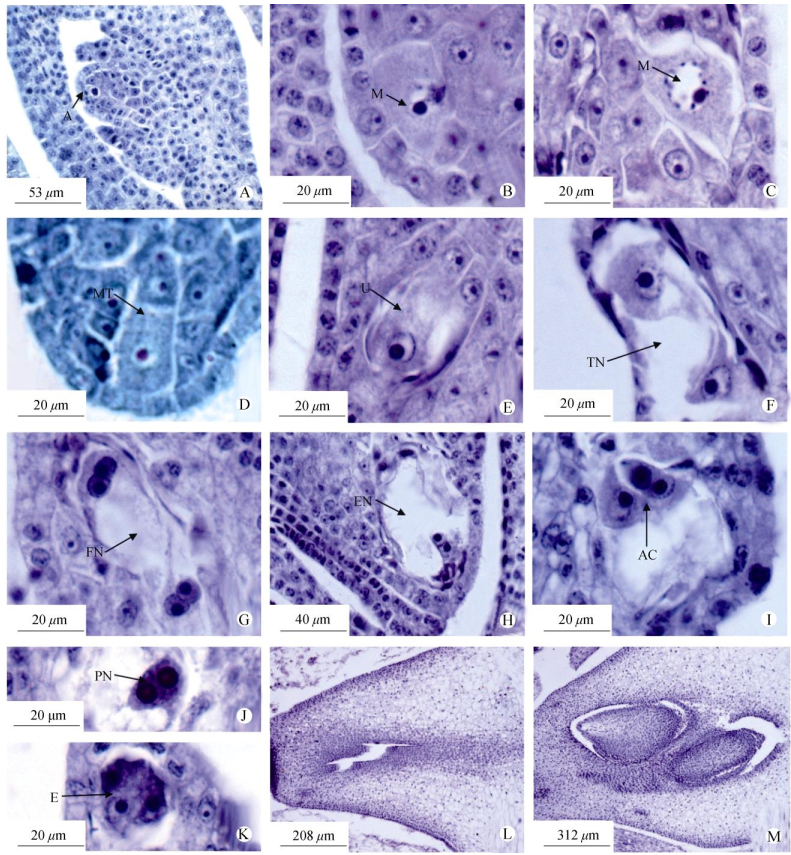
|
Fig. 2 Development of megasporogenesis and female gametophyte of Bambusa multiplex. A: Archesporial cell; B: Megaspore mother cell; C: Meiosis stage; D: Tetrad stage; E: Three megaspores near the chalazal degenerate and functional one near micropyle; F: Two-nucleus embryo sac; G: Four-nucleus embryo sac; H: Eight-nucleus embryo sac; I: Antipodal cells; J: Polar nuclei; K: Egg apparatus; L: Abortive ovary, no ovule in ovary; M: Two ovules in an ovary. |
After archesporial cell of Bambusa multiplex develop into functional megaspore, the female game- tophyte stage begins. The functional megaspore under- goes three times mitosis and develops into the female gametophyte successively (Fig. 2: F, G, H). The mature female gametophyte consists of 7 cells: 3 antipodal cells arranged transversely in the chalazal (Fig. 2: I), egg apparatus (1 egg cell and 2 synergid cells) posited near the micropylar end (Fig. 2: J) and a central cell (2 polar nucleus) remains in a central position in the embryo sac (Fig. 2: K).
2.4 Development relationship between pollen and ovaryIn order to find out the development relationship between the pollen and the ovary in the same floret, we collected 30 florets at different developmental stages. From Table 1, when the anther length is 0.5-1.5 mm, anthers development is at the stage of sporo- genous cell, while pistils are in the phase of arche- sporial cells. Thus, the development of ovary is slightly later than that of anther in early stage. But when the anthers' length is up to 4 mm, pistil and stamen development both enter meiosis stage, maintaining a high degree of consistency in the development. When male gametophyte matures (forms 3-nucleus pollen grain), the female gametophyte also matures (mainly forms eight-nucleate embryo). So Bambusa multiplex stamen and pistil are synchronization development, and there is no dichogamy phenomenon. Thus, Bambusa multiplex stamen and pistil development are homogamy. Meanwhile, when the florets open, anthers stretch out of the palea, so that the stigma with feather-like branches will receive pollen easily in field.
| Table 1 Development stage between pollen and ovary |
The florescence of bamboo plants is divided into short one and long one according to the characteristics of flowering. Longer florescence refers to flower two or more than two times for a whole year, and short florescence refers to flower only one time throughout a whole year. For Dendrocalamus sinicus, it belonged to long florescence bamboo species, because the flowering time is in one period from April to May and the other period from September to November. For Bambusa multiplex, the formation period of the reproductive structure is in spring, and it flowers only once in a whole year in Nanjing, so it belonged to short florescence bamboo species. The flowering characteristics of B. multiplex is similar with what has been described in other studies about the reproduction morphology of this species[12], and the florescence is the same as some bamboo species such as B. intermedia[20]and B. eutuldoides var. viridivittata[21].
Floral organ morphology has been one of the most important classification characteristics of bamboo plants. In the early period of the bamboo studies, Holttum[22-23] classified the ancient tropical woody bamboo system according to the style morphology. The pistils of most bamboos are refered to be consis- tent with the members of the grass family, which consist of stigma, short style and ovary. Usually the stigma is feather-like structure as bottlebrush, which has many small branches. And young stigma has short branches and mature stigma has more branches. The branching is variable in form between genera or between species within a genus. For example, in Shibataea chinensis the branching is very short when flowering[7]. Here in Bambusa multiplex, primordium initiated on the top of pistil then developed into 3 stigmas at the style's apex during the development of stigma. In bamboos, the style is usually a single column. Here we found in B. multiplex the style is shorter. Ovary is the basic and important structure. Its shape usually changes to egg-shaped with its growth. The morphology of pistle is similar with the other bamboo species such as Sasella kongosanensis 'Aureostriatus'[24].
The development of embryo sac of Bambusa multiplex is similar with those of other plants, such as Chimonobambusa marmorea[2], Dendrocalamus sinicus[4], Menstruocalamus sichuanensis[6], Shibataea chinensis[7], Oryza sativa[25].Bambusa multiplex pistil has one ovary with one ovule, which is anatropous, bitegmic and tenuinucellate. One archesporial cell grows directly into megasporocyte. A linear megaspores tetrad was found in rice[25], the same as megasporo- genesis in B. multiplex. Megaspore tetrads is linear, and then 3 megaspores degenerated, 1 megaspore differentiates into functional megaspore, which goes through successively two-nuclei stage, four-nuclei stage, and finally forms mature eight-nuclei embryo sac. The ovary structure is the same as basis characteristics of Gramineae. In the follow-up study, we need to investigate deeply the molecular mecha- nism of embryo sac development, so it is necessary to learn more about each development period of the embryo sac. The development relationship between pollen and ovary was studied in detail in this paper. So we can know the development phase of embryo sac only according to the anther length. Through compre- hensive research, we know stamen and pistil develop- ment of Bambusa multiplex are homogamy. When the flowers were in bloom, the expanding lodicule opened the lemma, the anthers with long filament stretched out of the lemma, so that the stigma received pollen easily in field.
The mature embryo sac of Bambusa multiplex includes 1 egg cell, 2 synergids, 1 central cell with 2 polar nucleus and 3 antipodal cells. Antipodal cells with abundant cytoplasm will degrade after fertili- zation. Therefore, antipodal cells are considered to be related to nutrition of the embryonic development. In Poaceae plants, the number of antipodal cells changes significantly. For example, the number of antipodal cells in rice is not regular, such as 3-5, 5-10, 10-15, 6-20[25-26]. However in our research, Bambusa multi- plex has a longer period with 3 antipodal cells. Synergids play the role in guiding male gametophyte to enter into female gametophyte in fertilization[27-28]. Yang et al. thought synergids had distinct filiform apparatus in rice[16], and synergids in Phyllostachys praecox are also with distinct filiform apparatus. Filiform apparatus may increase the contact area with the surround cells, and relate to the nutrient material between the integument and nucellus[29]. We also found that there are filamentous structure in Bambusa multiplex synergid.
Usually bamboos have a low seed rate mainly because of the abortive pollen and dysplasia embryo sac. Wang et al.[4] thought that low seed rate of Dendrocalamus sinicus was due to the characteristics of dichogamy. And, in the process of highly intensive management, most bamboo species often bloomed fragmentarily. So in the process of sexual reproduction normal fertilization opportunity is very limited. In this paper, it is homogamy in the same floret of B. multiplex, while homogamy is relatively uncommon in the angiosperms. It was recorded that the average fruiting rate of B. multiplex is only 9.8%. Low fruiting rate is mainly caused by abortive embryos, including the different development phase of male organs, female organs, pollination, fertilization, zygote dysplasia and endosperm, etc. While in our research, the development of anther is normal[30]. Here, Ovary developmental situation may be one of the reasons effecting on the setting rate. Bambusa multiplex stamens are well-developed, having no abnormalities in structures, but there are some anomalies during the development of some pistils: ① In early stage, the main abortion phenomenon is no ovules in the ovary (Fig. 2: L). ② Individual ovary has two ovules (Fig. 2: M), usually one ovule is large and the other small. These suggest that abnormal ovary development may be one of the reasons of low setting rate in B. multiplex.
| [1] | WU Z Y, RAVEN P H, Hong D Y. Flora of China, Volume 22[M]. Beijing: Science Press & St. Louis:Missouri Botanical Garden Press, 2006: 1-937. |
| [2] | HU C H, YU F G, PANG Y J. Observation and study on embryology of Chimonobambusa marmoreal[J]. J Bamboo Res, 1994, 13(4): 6-13, 69. |
| [3] | PANG Y J, YU F G, HU C H, et al. Preliminary observation on abnormal development of the stamens of Drepanostachyum micro-phyllum[J]. J Bamboo Res, 1994, 13(4): 42-46. |
| [4] | WANG S G, PU X L, DING Y L. The structures of reproductive organs and development of the female and male gametophyte of Dendro-calamus sinicus[J]. Bull Bot Res, 2006, 26(3): 270-274. DOI:10.3969/j.issn.1673-5102.2006.03.006.(inChinese) |
| [5] | HUANG J Q, HUANG H H, HE F J, et al. The Formation of microspore and the development of male gametophyte of Phyllostachys praecox[J]. J Bamboo Res, 1999, 18(3): 55-58. |
| [6] | LIN S Y, HAO J J, XIN H, et al. The megasporogenesis, microspo-rogenesis and the development of their female and male gametophyte in Menstruocalamus sichuanensis[J]. J Nanjing For Univ (Nat Sci), 2009, 33(3): 9-12. DOI:10.3969/j.issn.1000-2006.2009.03.003.(inChinese) |
| [7] | LIN S Y. Studies on the reproductive biology of Shibataea chinensis and Arundinaria simonii f. albostriatus[D]. Nanjing: Nanjing Forestry University, 2009: 1-125. doi: 10.7666/d.y1645758.(inChinese) |
| [8] | LIN S Y, DING Y L. Development of the male and female gameto-phytes in Shibataea chinensis (Bambusoideae)[J]. Acta Bot Boreali-Occid Sin, 2012, 32(5): 907-914. DOI:10.3969/j.issn.1000-4025.2012.05.010.(inChinese) |
| [9] | LIN S Y, DING Y L. Observations on megasporogenesis, micro-sporogenesis and development of the male and female gametophytes of Arundinaria simonii f. heterophylla[J]. Sci Silv Sin, 2013, 49(8): 168-175. DOI:10.11707/j.1001-7488.20130824 |
| [10] | Yuan J L, Guo G P, Yue J J, et al. Features of DNA Methylation during the flowering process of Bambusa multiplex[J]. Acta Bot Boreali-Occid Sin, 2012, 32(1): 60-66. |
| [11] | HUANG C Q, HUANG T, LIU W, et al. The determination of frost resistance of 20 kinds of ornamental cluster bamboo[J]. J Hunan City Univ (Nat Sci), 2013, 22(2): 59-62. DOI:10.3969/j.issn.1672-7304.2013.02.016.(inChinese) |
| [12] | YUAN J L, GU X P, YUE J J, et al. Flowering biology and crossing of Bambusa multiplex[J]. Sci Silv Sin, 2011, 47(8): 61-66. DOI:10.11707/j.1001-7488.20110810.(inChinese) |
| [13] | YUAN J L, YUE J J, WU X L, et al. Medium optimization for callus proliferation of Bambusa multiplex[J]. Bull Bot Res, 2010, 30(5): 562-567. |
| [14] | YUAN J L, Gu X P, Li L B, et al. Callus induction and plantlet regeneration of Bambusa multiplex[J]. Sci Silv Sin, 2009, 45(3): 35-39. DOI:10.3321/j.issn:1001-7488.2009.03.007.(inChinese) |
| [15] | McCLURE F A. translated. Reproductive phase of bamboos, Volume 1[J]. J Bamboo Res, 1982, 1(2): 104-116. |
| [16] | McCLURE F A. HONG X X, translated. Reproductive phase of bamboos, Volume 2[J]. J Bamboo Res, 1983, 2(2): 119-136. |
| [17] | YUAN X L, LIN X C, LIN R, et al. Advances in the studies of bamboo flowering[J]. J Bamboo Res, 2007, 26(1): 6-9, 14. DOI:10.3969/j.issn.1000-6567.2007.01.002.(inChinese) |
| [18] | LI Z L. Botanical Microtechnique[M]. 2nd ed. Beijing: Science Press, 1987: 1-170. |
| [19] | GERSTERBERGER P, LEINS P. Rasterelektronenmikroskopische untersuchungen an blütenknospen von Physalis philadelphica (Solanaceae)[J]. Plant Biol, 1978, 91(1): 381-387. DOI:10.1111/j.1438-8677.1978.tb03660.x |
| [20] | WANG Y J, LUO J, CHEN N N, et al. CHEN N N, et al. Floral Morphology and Development of Female and Male Gametophyte of Bambusa intermedia Hsueh et Yi[J]. Bull Bot Res, 2017, 37(4): 492-498. DOI:10.7525/j.issn.1673-5102.2017.04.002.(inChinese) |
| [21] | TANG G J, YANG J M, DING Y L, et al. Studies on the flower morphology and structure in Bambusa eutuldoides McClure var. viridivittata (W. T. Lin) Chia[J]. J Nanjing For Univ (Nat Sci), 2016, 40(2): 71-75. DOI:10.3969/j.issn.1000-2006.2016.02.012.(inChinese) |
| [22] | HOLTTUM R E. The classification of Malayan bamboos[J]. J Arn Arb, 1946, 27(4): 340-346. |
| [23] | HOLTTUM R E. The classification of Bamboos[J]. Phytomorphology, 1956, 6: 73-90. |
| [24] | LIN S Y, FAN T T, JIANG M Y, et al. The revision of scientific names for three dwarf bamboo species (cultivar) based on the floral morpho-logy[J]. J Nanjing For Univ (Nat Sci), 2017, 41(1): 189-193. DOI:10.3969/j.issn.1000-2006.2017.01.029.(inChinese) |
| [25] | YANG H Y. Rice Reproductive Biology[M]. Hangzhou: Zhejiang University Press, 2005: 19-69. |
| [26] | LI J. Studies on the reproductive biology of Bambusa multiplex[D]. Nanjing: Nanjing Forestry University, 2013: 1-67. (in Chinese) |
| [27] | WANG Y Y, TIAN H Q. Development and function of the synergid cell of angiosperms[J]. Chin Bull Bot, 2009, 44(4): 506-514. DOI:10.3969/j.issn.1674-3466.2009.04.013.(inChinese) |
| [28] | YANG W C, SHI D Q. Research advances in plant female gameto-genesis[J]. Chin Bull Bot, 2007, 24(3): 302-310. DOI:10.3969/j.issn.1674-3466.2007.03.006.(inChinese) |
| [29] | YUAN X L. Studies on florescence biological characteristics and flower developmental anatomy of Phyllostachys praecox[D]. Hang-zhou: Zhejiang Agricultural and Forest University, 2007: 1-52. (in Chinese) |
| [30] | LIN S Y, LI J, ZHAO R, et al. The development of flowering bud differentiation and male gametophyte of Bambusa multiplex[J]. J Nanjing For Univ (Nat Sci), 2015, 39(4): 51-56. DOI:10.3969/j.issn.1000-2006.2015.04.009.(inChinese) |
 2018, Vol. 26
2018, Vol. 26


