2. 武汉科技大学化学与化工学院, 武汉 430081
2. School of Chemical Engineering and Technology, Wuhan University of Science and Technology, Wuhan 430081, China
溪黄草药材为唇形科(Lamiaceae)植物线纹香茶菜(Isodon lophanthoides)和溪黄草(I. serra)的干燥地上部分,是我国药典收载成方制剂消炎利胆片和胆石通胶囊的组成之一[1]。线纹香茶菜和溪黄草在广东、广西、福建、江西等省区有种植,以满足消炎利胆片、胆石通胶囊、十味溪黄草颗粒等中成药及溪黄草冲剂、溪黄草袋泡茶、溪黄八珍茶等保健品的生产需求,在民间还作为药茶或煲汤料, 用于防治急性黄疸性肝炎、急性胆囊炎、肠炎等病症[2]。
对线纹香茶菜和溪黄草的化学成分已有较多的报道,从中分离鉴定的成分有二萜、三萜、黄酮、苯丙素、有机酸、甾醇等结构类型[2-4]。为阐明线纹香茶菜和溪黄草化学物质基础的异同,我们对同一种植基地的线纹香茶菜和溪黄草地上部分的化学成分进行了深入研究。前文报道了从线纹香茶菜纤花变种中分得的16个二萜类、13个酚类和11个其它类型化合物的结构及其体外细胞毒、抗氧化和抗菌活性,其中11个为新化合物,24个已知化合物为首次从该植物中报道[5-8]。本文报道从溪黄草中分得的2个苯丙素类、4个大柱香波龙烷类、1个生物碱类和2个烷基糖苷类化合物。
1 材料和方法 1.1 材料青岛谱科分离材料公司柱层析硅胶;烟台江友硅胶开发公司薄层色谱硅胶板;AB公司葡聚糖凝胶LH-20。
溪黄草地上部分于2011年10月采自广州白云山和记黄埔中药有限公司在广东英德市大湾镇的种植基地,由中国科学院华南植物园叶华谷研究员鉴定为Isodon serra。
1.2 仪器岛津公司LC-6AD高效液相色谱仪和RID-10A示差检测器;利穗科技(苏州)公司EZ Purifier 100中压液相色谱仪;AB公司MDS SCIEX API 2000 LC-MS/MS质谱仪;PE公司343型旋光仪;Bruker Ascend-500核磁共振仪,以溶剂峰为参照。
1.3 提取和分离将新鲜溪黄草地上部分切段,阴干,粉碎。取粉末10.0 kg,用95%乙醇和50%乙醇各浸泡2次,溶剂体积依次为50、40、40和40 L,每次2 d, 合并滤液,减压浓缩至干,得乙醇提取物760 g。提取物用3 L蒸馏水溶解,倒入分液漏斗,加石油醚脱色3次,每次3 L。水液用乙酸乙酯和正丁醇各萃取4次,每次3 L,合并萃取液,减压浓缩至干,得乙酸乙酯萃取物163 g和正丁醇萃取物171 g。
乙酸乙酯萃取物经硅胶柱层析,以三氯甲烷-甲醇混合液[10:0~7:3 (V/V, 下同)]洗脱,流份经薄层板检查,合并为17个组分(E1~E17)。E9 (23.2 g)经硅胶柱层析,以石油醚-丙酮(9:1~6:4)洗脱, 合并为8个亚组份(E9-1~E9-8)。E9-5经中压液相色谱分离,以甲醇-水(4:6~8:2)洗脱,4:6洗脱物用薄层板制备,得化合物6 (7.5 mg)。E10 (75 g)经硅胶柱层析,以石油醚-丙酮(9:1~4:6)洗脱, 合并为10个亚组分(E10-1~E10-10)。E10-5经中压液相色谱分离,以甲醇-水(3:7~6:4)洗脱;3:7洗脱物经高效液相色谱纯化,以甲醇-水(39:61)为流动相,流速5 mL min-1,得化合物7 (tR=52.2 min, 9.3 mg)。E10-10经硅胶柱层析,以三氯甲烷-甲醇(10:0~85:15)洗脱,合并为7个次组分(E10-10-1~ E10-10-7)。E10-10-1经中压液相色谱分离,以甲醇-水(3:7~6:4)洗脱,4:6洗脱物经葡聚糖凝胶LH-20柱色谱分离,以甲醇洗脱,得化合物2 (13.4 mg)。正丁醇萃取物经硅胶柱层析,以三氯甲烷-甲醇(8:2~5:5)洗脱,合并为10个组分(B1~B10)。B1 (29.9 g)经硅胶柱层析,以石油醚-丙酮(9:1~4:6)洗脱, 合并为11个亚组分(B1-1~B1-11)。B1-7经中压液相色谱分离,以甲醇-水(5:5~87:13)洗脱,合并为11个次组分(B1-7-1~B1-7-11)。B1-7-1经高效液相色谱纯化,以甲醇-水(44:56)为流动相,流速为5 mL min-1,得化合物3 (tR=26.2 min, 11.2 mg)。B1-7-11经LH-20柱色谱分离,以甲醇洗脱,得化合物5 (1.5 mg)。B2 (32.7 g)经硅胶柱层析,以三氯甲烷-甲醇(9:1~7:3)洗脱,合并为10个亚组分(B2-1~B2-10)。B2-3经中压液相色谱分离,以甲醇-水(5:5~87:13)洗脱,合并为8个次组分(B2-3-1~ B2-3-8)。B2-3-4经LH-20柱色谱分离,再经高效液相色谱纯化,以甲醇-水(49:51)为流动相,流速为5 mL min-1,得化合物1 (tR=47.3 min, 61.2 mg)。B2-5经硅胶柱层析,再经高效液相色谱纯化,以甲醇-水(1:9)为流动相,流速5 mL min-1,得化合物8 (tR=18.0 min, 15.1 mg)和9 (tR=32.8 min, 10.8 mg)。B6 (20.5 g)经硅胶柱层析,以三氯甲烷-甲醇(9:1~ 7:3)洗脱,合并为7个亚组分(B6-1~B6-7)。B6-6经硅胶柱层析,再经高效液相色谱纯化,以甲醇-水(18:82)为流动相,流速5 mL min-1,得化合物4 (tR=82.7 min, 46.0 mg)。
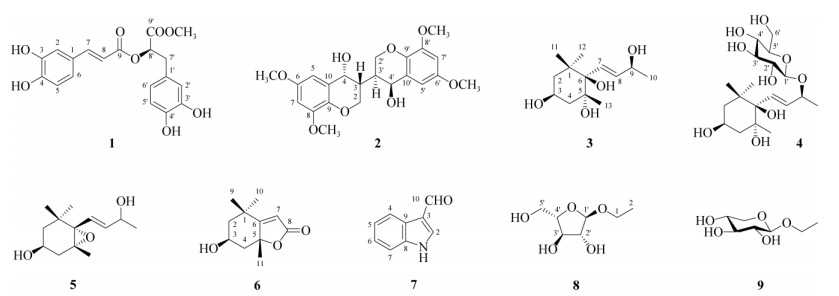
1.4 结构鉴定化合物1 无色粉末(甲醇);[α]D20 + 82.9 (c 0.58, MeOH); ESI-MS m/z: 396.7 [M + Na]+, 770.9 [2M + Na]+, 372.5 [M-H]-, 409.1 [M + Cl]-, 分子量374.1, 分子式C19H18O8; 1H NMR (C5D5N, 500 MHz): δ 7.56 (1H, d, J = 1.9 Hz, H-2), 7.20 (1H, d, J = 8.1 Hz, H-5), 7.11 (1H, dd, J = 8.1, 1.9 Hz, H-6), 8.01 (1H, d, J = 15.8 Hz, H-7), 6.62 (1H, d, J = 15.8 Hz, H-8), 7.39 (1H, d, J = 2.1 Hz, H-2′), 7.24 (1H, d, J = 8.0 Hz, H-5′), 6.93 (1H, dd, J = 8.0, 2.1 Hz, H-6′), 3.35 (1H, dd, J = 14.3, 5.1 Hz, Ha-7′), 3.31 (1H, dd, J = 14.3, 7.8 Hz, Hb-7′), 5.72 (1H, dd, J = 7.5, 5.1 Hz, H-8′), 3.64 (3H, s, OCH3); 13C NMR (C5D5N, 125 MHz): δ 127.1 (C-1), 116.5 (C-2), 148.2 (C-3), 151.3 (C-4), 117.1 (C-5), 122.9 (C-6), 147.6 (C-7), 114.4 (C-8), 167.6 (C-9), 128.7 (C-1′), 118.4 (C-2′), 147.8 (C-3′), 146.9 (C-4′), 117.2 (C-5′), 121.6 (C-6′), 37.9 (C-7′), 74.4 (C-8′), 171.4 (C-9′), 52.5 (OCH3)。以上数据与文献[9]报道的(CD3OD)基本一致,故鉴定为迷迭香酸甲酯。
化合物2 淡黄色粉末(甲醇);[α]D20 0 (c 0.67, MeOH); ESI-MS m/z: 441.2 [M + Na]+, 453.8 [M + Cl]-, 分子量418, 分子式C22H26O8; 1H NMR (CD3OD, 500 MHz): δ 4.26 (2H, dd, J = 9.0, 4.7 Hz, H-2a, 2′a), 3.88 (2H, br d, J = 9.0 Hz, H-2e, 2′e), 3.14 (2H, m, H-3, 3′′), 4.71 (2H, br s, H-4, 4′), 6.65 (4H, br s, H-5, 7, 5′, 7′′), 3.84 (12H, s, OCH3×4); 13C NMR (CD3OD, 125 MHz): δ 72.8 (C-2, 2′), 55.5 (C-3, 3′), 87.6 (C-4, 4′), 104.5 (C-5, 7, 5′, 7′), 149.3 (C-6, 6′, 8, 8′), 136.2 (C-9, 9′), 133.1 (C-10, 10′), 56.8 (OCH3×4)。以上数据与文献[10]报道(CDCl3)的基本一致,故鉴定为3, 3′-双(3, 4-二氢-4-羟基-6, 8-二甲氧基-2H-1-苯并吡喃)。
|
图 1 化合物1~9的结构 Fig. 1 Structures of compounds 1-9 |
化合物3 无色粉末(甲醇);[α]D20 –22.8 (c 0.56, MeOH); ESI-MS m/z: 267.3 [M + Na]+, 511.4 [2M + Na]+, 279.4 [M + Cl]-, 分子量244, 分子式C13H24O4; 1H NMR (C5D5N, 500 MHz): δ 2.59 (1H, dd, J = 12.2, 11.7 Hz, Ha-2), 2.05 (1H, dd, J = 12.2, 4.3 Hz, He-2), 4.91 (1H, tt, J = 11.7, 4.3 Hz, H-3), 2.48 (1H, dd, J = 12.2, 11.7 Hz, Ha-4), 2.50 (1H, dd, J = 11.7, 4.3 Hz, He-4), 6.84 (1H, d, J = 15.8 Hz, H-7), 6.51 (1H, dd, J = 15.8, 6.1 Hz, H-8), 4.77 (1H, qd, J = 6.1, 6.4 Hz, H-9), 1.50 (3H, d, J = 6.4 Hz, H3-10), 1.66 (3H, s, H3-11), 1.64 (3H, s, H3-12), 1.27 (3H, s, H3-13); 13C NMR (C5D5N, 125 MHz): δ 40.9 (C-1), 47.7 (C-2), 64.7 (C-3), 47.4 (C-4), 77.5 (C-5), 79.0 (C-6), 131.0 (C-7), 136.9 (C-8), 68.8 (C-9), 25.5 (C-10), 28.3 (C-11), 26.9 (C-12), 28.3 (C-13)。以上数据与文献[11-12]报道的一致,故鉴定为7-大柱香波龙烯-3, 5, 6, 9-四醇。
化合物4 无色粉末(甲醇);[α]D20 –55.3 (c 1.25, MeOH); ESI-MS m/z: 429.4 [M + Na]+, 835.4 [2M + Na]+, 405.5 [M-H]-, 分子量406, 分子式C19H34O9; 1H NMR (C5D5N, 500 MHz): δ 2.52 (1H, dd, J = 12.2, 11.8 Hz, Ha-2), 2.01 (1H, dd, J = 12.2, 4.2 Hz, He-2), 4.91 (1H, tt, J = 11.8, 4.2 Hz, H-3), 2.42 (1H, dd, J = 12.2, 11.8 Hz, Ha-4), 2.48 (1H, dd, J = 11.7, 4.3 Hz, He-4), 6.70 (1H, d, J = 15.9 Hz, H-7), 6.50 (1H, dd, J = 15.9, 7.4 Hz, H-8), 4.73 (1H, qd, J = 6.2, 7.4 Hz, H-9), 1.40 (3H, d, J = 6.2 Hz, H3-10), 1.67 (3H, s, H3-11), 1.64 (3H, s, H3-12), 1.19 (3H, s, H3-13), 4.94 (1H, d, J = 7.7 Hz, H-1′), 4.22 (1H, dd, J = 7.7, 8.8 Hz, H-2′), 4.03 (1H, dd, J = 8.8, 9.2 Hz, H-3′), 3.94 (1H, m, H-5), 4.57 (1H, dd, J = 11.6, 2.3 Hz, Ha-6′), 4.23 (1H, dd, J = 11.6, 6.4 Hz, Hb-6′); 13C NMR (125 MHz, C5D5N): δ 40.6 (C-1), 47.4 (C-2), 64.6 (C-3), 47.0 (C-4), 77.4 (C-5), 78.6 (C-6), 133.3 (C-7), 134.5 (C-8), 78.9 (C-9), 22.1 (C-10), 28.6 (C-11), 26.8 (C-12), 28.0 (C-13), 103.2 (C-1′), 75.6 (C-2′), 78.0 (C-3′), 72.4 (C-4′), 78.7 (C-5′), 63.2 (C-6′)。以上数据与文献[13]报道(CD3OD)的基本一致,故鉴定为7-大柱烯-3, 5, 6, 9-四醇9-O-β-D-葡萄糖苷。
化合物5 无色粉末(甲醇);[α]D20 –68.5 (c 0.15, MeOH); ESI-MS m/z: 249.5 [M + Na]+, 分子量226, 分子式C13H22O3; 1H NMR (C5D5N, 500 MHz): δ 2.62 (1H, dd, J = 14.2, 11.6 Hz, Ha-2), 1.97 (1H, dd, J = 14.2, 8.8 Hz, He-2), 4.28 (1H, m, H-3), 1.54 (1H, dd, J = 12.9, 10.4 Hz, Ha-4), 1.89 (1H, dd, J = 12.9, 3.3 Hz, He-4), 6.22 (1H, d, J = 17.4 Hz, H-7), 5.48 (1H, dd, J = 17.5, 6.4 Hz, H-8), 4.67 (1H, qd, J = 6.4, 6.4 Hz, H-9), 1.46 (3H, d, J = 6.4 Hz, H3-10), 1.18 (3H, s, H3-11), 1.11 (3H, s, H3-12), 1.29 (3H, s, H3-13); 13C NMR (C5D5N, 125 MHz): δ 35.2 (C-1), 48.2 (C-2), 63.3 (C-3), 42.0 (C-4), 69.8 (C-5), 72.5 (C-6), 124.5 (C-7), 139.7 (C-8), 67.5 (C-9), 24.6 (C-10), 25.3 (C-11), 29.8 (C-12), 20.3 (C-13)。以上数据与文献[14]报道(CD3OD)的基本一致,故鉴定为5, 6-环氧-7-大柱烯-3, 9-二醇。
化合物6 白色粉末(甲醇);[α]D20 –73.6 (c 0.50, MeOH); ESI-MS m/z: 218.9 [M + Na]+, 415.1 [2M + Na]+, 195.2 [M-H]-, 分子量196, 分子式C11H16O3; 1H NMR (C5D5N, 500 MHz): δ 2.59 (1H, dt, J = 13.4, 2.6 Hz, H-2), 1.75 (1H, dd, J = 13.4, 3.9 Hz, H-2), 5.85 (1H, m, H-3), 2.03 (1H, dt, J = 14.2, 2.6 Hz, H-4), 1.40 (1H, dd, J = 14.2, 3.7 Hz, H-4), 6.63 (1H, s, H-7), 1.46 (3H, d, J = 6.4 Hz, H3-9), 1.13 (1H, s, H3-10), 1.51 (3H, s, H3-11); 13C NMR (C5D5N, 125 MHz): δ 36.6 (C-1), 48.1 (C-2), 66.3 (C-3), 46.8 (C-4), 87.4 (C-5), 183.3 (C-6), 113.4 (C-7), 172.2 (C-8), 26.9 (C-9), 31.0 (C-10), 27.7 (C-11)。以上数据与文献[5]报道(CDCl3)的基本一致,故鉴定为(-)-燕麦草内酯。
化合物7 白色粉末(甲醇);ESI-MS m/z: 168.3 [M + Na]+, 312.9 [2M + Na]+, 143.9 [M-H]-, 170.8 [M + Cl]-, 分子量145, 分子式C9H7NO; 1H NMR (C5D5N, 500 MHz): δ 8.24 (1H, s, H-2), 8.77 (1H, d, J = 7.5 Hz, H-4), 7.40 (1H, br t, J = 7.5 Hz, H-5), 7.38 (1H, br t, J = 7.5 Hz, H-6), 7.60 (1H, d, J = 7.5 Hz, H-7), 10.32 (1H, s, CHO); 13C NMR (C5D5N, 125 MHz): δ 138.4 (C-2), 120.2 (C-3), 124.5 (C-4), 123.2 (C-5), 122.5 (C-6), 113.2 (C-7), 138.7 (C-8), 125.9 (C-9), 185.5 (CHO)。以上数据与文献[15]报道(CD3OD)的基本一致,故鉴定为3-醛基吲哚。
化合物8 白色粉末(甲醇);[α]D20 +40.2 (c 0.75, MeOH); ESI-MS m/z: 201.2 [M + Na]+, 378.8 [2M + Na]+, 177.0 [M-H]-, 分子量178, 分子式C7H14O5; 1H NMR (C5D5N, 500 MHz): δ 3.52 (1H, dq, J = 9.6, 7.1 Hz, Ha-1), 3.95 (3H, dq, J = 9.6, 7.1 Hz, Hb-1), 1.15 (3H, t, J = 7.1 Hz, H3-2), 5.47 (1H, d, J = 2.0 Hz, H-1′), 4.85 (1H, m, H-2′), 4.83 (1H, m, H-3′), 4.69 (1H, ddd, J = 7.1, 4.8, 3.0 Hz, H-4′), 4.37 (1H, dd, J = 11.9, 3.0 Hz, Ha-5′), 4.24 (1H, dd, J = 11.9, 4.8 Hz, Hb-5′); 13C NMR (C5D5N, 125 MHz): δ 63.7 (C-1), 15.8 (C-2), 109.7 (C-1′), 84.1 (C-2′), 78.9 (C-3′), 85.7 (C-4′), 63.1 (C-5′)。以上数据与文献[16]报道的一致,故鉴定为乙基α-L-呋喃阿拉伯糖苷。
化合物9 白色粉末(甲醇);[α]D20 -56.5 (c 0.54, MeOH); ESI-MS m/z: 201.2 [M + Na]+, 378.9 [2M + Na]+, 177.2 [M-H]-, 213.0 [M + Cl]-, 分子量178, 分子式C7H14O5; 1H NMR (C5D5N, 500 MHz): δ 3.69 (1H, dq, J = 9.5, 7.0 Hz, Ha-1), 4.08 (3H, dq, J = 9.5, 7.0 Hz, Hb-1), 1.20 (3H, t, J = 7.0 Hz, H3-2), 4.70 (1H, d, J = 7.6 Hz, H-1′), 4.00 (1H, dd, J = 8.8, 7.6 Hz, H-2′), 4.16 (1H, t, J = 8.8 Hz, H-3′), 4.24 (1H, m, H-4′), 4.35 (1H, dd, J = 11.3, 5.3 Hz, Ha-5′), 3.67 (1H, br d, J = 11.3 Hz, Hb-5′); 13C NMR (C5D5N, 125 MHz): δ 65.1 (C-1), 15.7 (C-2), 105.3 (C-1′), 75.1 (C-2′), 78.5 (C-3′), 71.2 (C-4′), 67.4 (C-5′)。以上数据与文献[16]报道的一致,故鉴定为乙基β-D-木糖苷。
2 结果和讨论利用色谱分离方法从溪黄草地上部分的乙醇提取物中分得9个化合物,并通过光谱数据分析鉴定了他们的结构,分别为迷迭香酸甲酯(1)、3, 3′-双(3, 4-二氢-4-羟基-6, 8-二甲氧基-2H-1-苯并吡喃) (2)、7-大柱香波龙烯-3, 5, 6, 9-四醇(3)、7-大柱香波龙烯-3, 5, 6, 9-四醇9-O-β-D-葡萄糖苷(4)、5, 6-环氧-7-大柱香波龙烯-3, 9-二醇(5)、(-)-黑麦草内酯(6)、3-醛基吲哚(7)、乙基α-L-呋喃阿拉伯糖苷(8)和乙基β-D-木糖苷(9)。迷迭香酸甲酯(1)以外的化合物均为首次从该种植物中报道。
迷迭香酸甲酯(1)能抑制人全血的氧化迸发(最大半数抑制浓度IC50=11.0 μg mL-1),通过抑制白细胞介素-2 (IL-2)的产生而抑制人T-细胞增殖(IC50 < 3.1 μg mL-1),还可抑制IL-4 (IC50=6.5 μg mL-1)的产生和增加肿瘤坏死因子-α (TNF-α)的产生;分子对接实验表明,它是强有力的IL-2抑制剂,具有免疫调节作用[17]。迷迭香酸甲酯还具有抑制黄嘌呤氧化酶(IC50=26.6 μmol L-1)和基质金属蛋白酶-1 (MMP-1) (IC50=14.7 μmol L-1)活性[18-19]。(-)-黑麦草内酯(6)可减少阿霉素诱导的衰老人皮肤成纤维细胞中与衰老相关的β-半乳糖苷酶活性、p21蛋白水平和活性氧水平,还可减弱复制性衰老人皮肤成纤维细胞中的β-半乳糖苷酶活性,表明它可延缓人皮肤成纤维细胞的衰老,有望开发成用于改善组织衰老或衰老相关疾病的膳食补充剂或化妆品[20]。黑麦草内酯对人结肠癌Caco-2细胞的增殖具有抑制作用(IC50=30 μg mL-1)[21];与雌激素受体α的活化抑制作用相比,它更多地抑制1 nmol L-1 17β-雌二醇诱导的雌激素受体α的活化[22],还具有中等强度的抑制胆碱酯酶活性(IC50=7.57 μg mL-1)[23]。3-醛基吲哚(7)对恰加斯病的病原体克氏锥虫(Trypanosoma cruzi)显示出一定的抑制活性(IC50= 26.9 μmol L-1)[24]。由此可见,迷迭香酸甲酯和黑莓草内酯参与了溪黄草的保肝、免疫抑制、抗炎、抗肿瘤作用。
| [1] |
Chinese Pharmacopoeia Commision. Chinese Pharmacopoeia[M]. Beiing:China Medical Science Press, 2015, Ⅰ:1263-1264, 1416-1417, Ⅳ:424.
国家药典委员会. 中华人民共和国药典[M]. 北京: 中国医药科技出版社, 2015, 一部: 1263-1264, 1416-1417, 四部: 424. |
| [2] |
HUANG S S. Advances in a Chinese medicine 'Xihuangcao'[J].
Pharm Today, 2016, 26(5): 365-368. 黄珊珊. 中药溪黄草研究进展[J]. 今日药学, 2016, 26(5): 365-368. |
| [3] | LIN L Z, DONG Y, YANG B, et al. Chemical constituents and biological activity of Chinese medicinal herb 'Xihuangcao'[J]. Comb Chem High Throughput Screen, 2011, 14(8): 720-729. DOI:10.2174/138620711796504352 |
| [4] |
LIU F L, CHEN D J, FENG X L, et al. Chemical constituents from Isodon serra (Maxim.) Hara[J].
Trad Chin Drug Res Clin Pharmacol, 2016, 27(2): 242-245. 刘方乐, 陈德金, 冯秀丽, 等. 溪黄草的化学成分研究[J]. 中药新药与临床药理, 2016, 27(2): 242-245. |
| [5] |
LIANG Y G, XU X Y, XIE H H, et al. Chemical constituents from Isodon lophanthoides var. graciliflora[J].
J Trop Subtrop Bot, 2010, 18(5): 564-568. 梁耀光, 徐新亚, 谢海辉, 等. 细花线纹香茶菜的化学成分研究[J]. 热带亚热带植物学报, 2010, 18(5): 564-568. DOI:10.3969/j.issn.1005-3395.2010.05.015 |
| [6] | ZHOU W T, XIE H H, WU P, et al. Abietane diterpenoids from Isodon lophanthoides var. graciliflorus and their cytotoxicity[J]. Food Chem, 2013, 136(2): 1110-1116. DOI:10.1016/j.foodchem.2012.08.015 |
| [7] | LIANG Y G, XIE H H, WU P, et al. Podocarpane, isopimarane, and abietane diterpenoids from Isodon lophanthoides var. graciliflorus[J]. Food Chem, 2013, 136(3/4): 1177-1182. DOI:10.1016/j.foodchem.2012.09.084 |
| [8] | ZHOU W T, XIE H H, XU X Y, et al. Phenolic constituents from Isodon lophanthoides var. graciliflorus and their antioxidant and anti-bacterial activities[J]. J Funct Foods, 2014, 6: 492-498. DOI:10.1016/j.jff.2013.11.015 |
| [9] | WOO E R, PIAO M S. Antioxidative constituents from Lycopus lucidus[J]. Arch Pharm Res, 2004, 27(2): 173-176. DOI:10.1007/BF02980102 |
| [10] | DIEN P H, LIN L G, TANG C P, et al. Bisbenzopyrans and alkaloids from the roots of Stemona cochinchinensis[J]. Nat Prod Res, 2008, 22(10): 915-920. DOI:10.1080/14786410701642771 |
| [11] | ZHANG Z, ZHANG W, JI Y P, et al. Gynostemosides A-E, mega-stigmane glycosides from Gynostemma pentaphyllum[J]. Phyto-chemistry, 2010, 71(5/6): 693-700. DOI:10.1016/j.phytochem.2009.12.017 |
| [12] |
JIANG J W, ZHAO S X, SHI S M. Discussion on translation of ionone and megastigmane[J].
Chin Trad Herb Drugs, 2011, 42(6): 1243-1244. 江纪武, 赵守训, 时圣明. Ionone和megastigmane译名商榷[J]. 中草药, 2011, 42(6): 1243-1244. |
| [13] | OTSUKA H, HIRATA E, SHINZATO T, et al. Stereochemistry of megastigmane glucosides from Glochidion zeylanicum and Alangium premnifolium[J]. Phytochemistry, 2003, 62(5): 763-768. DOI:10.1016/S0031-9422(02)00614-3 |
| [14] | D'ABROSCA B, DELLAGRECA M, FIORENTINO A, et al. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui[J]. Phytochemistry, 2004, 65(4): 497-505. DOI:10.1016/j.phytochem.2003.11.018 |
| [15] |
TANG B Q, YANG T T, YANG W Q, et al. Chemical constituents in leaves of Morus atropurpurea and their α-glucosidase activity[J].
Chin Trad Herb Drugs, 2013, 44(22): 3109-3113. 唐本钦, 杨婷婷, 杨文强, 等. 广东桑叶化学成分及其α-葡萄糖苷酶活性研究[J]. 中草药, 2013, 44(22): 3109-3113. DOI:10.7501/j.issn.0253-2670.2013.22.003 |
| [16] | XIE H H, XU X Y, DAN Y, et al. Alkyl glycosides from mycelial cultures of Dichomitus squalens[J]. Chin J Nat Med, 2009, 7(5): 390-393. DOI:10.3724/SP.J.1009.2009.00390 |
| [17] | MESAIK M H, JABEEN A, HALIM S A, et al. In silico and in vitro immunomodulatory studies on compounds of Lindelofia stylosa[J]. Chem Biol Drug Des, 2012, 79(3): 290-299. DOI:10.1111/j.1747-0285.2011.01310.x |
| [18] | HUO L N, WANG W, ZHANG C Y, et al. Bioassay-guided isolation and identification of xanthine oxidase inhibitory constituents from the leaves of Perilla frutescens[J]. Molecules, 2015, 20(10): 17848-17859. DOI:10.3390/molecules201017848 |
| [19] | YUAN H, LU W Q, WANG L Y, et al. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1(MMP-1)[J]. Eur J Med Chem, 2013, 62: 148-157. DOI:10.1016/j.ejmech.2012.09.047 |
| [20] | YANG H H, HWANGBO K, ZHENG M S, et al. Inhibitory effects of (−)-loliolide on cellular senescence in human dermal fibroblasts[J]. Arch Pharm Res, 2015, 38(5): 876-884. DOI:10.1007/s11418-014-0817-0 |
| [21] | MACHADO F B, YAMAMOTO R E, ZANOLI K, et al. Evaluation of the antiproliferative activity of the leaves from Arctium lappa by a bioassay-guided fractionation[J]. Molecules, 2012, 17(2): 1852-1859. DOI:10.3390/molecules17021852 |
| [22] | HONG Y H, WANG S C, HSU C, et al. Phytoestrogenic compounds in alfalfa sprout (Medicago sativa) beyond coumestrol[J]. J Agric Food Chem, 2011, 59(1): 131-137. DOI:10.1021/jf102997p |
| [23] | FANG Z, JEONG S Y, JUNG H A, et al. Anticholinesterase and antioxidant constituents from Gloiopeltis furcate[J]. Chem Pharm Bull, 2010, 58(9): 1236-1239. DOI:10.1248/cpb.58.1236 |
| [24] | MART NEZ-LUIS S, G MEX J F, SPADAFORA C, et al. Antitry-panosomal alkaloids from the marine bacterium Bacillus pumilus[J]. Molecules, 2012, 17(9): 11146-11155. DOI:10.3390/molecules170911146 |
 2018, Vol. 26
2018, Vol. 26


