草莓(Fragaria×ananassa Duch.)为蔷薇科草莓属多年生草本,别名凤梨草莓,系威州草莓(F. virgirniana Duch.)与智利草莓[F. chiloensis (L.) Ehrh.]杂交而成的八倍体(2n=56),野生草莓为二倍体或四倍体。草莓在温带和亚热带地区广泛栽培.二十世纪初引入我国,目前我国草莓栽培面积和产量均居世界第一。草莓果实外形美观、色泽鲜艳、清香可口,富含维生素、矿物质、膳食纤维等营养性成分,还富含多酚类等次生代谢产物,具有预防心脑血管疾病、预防贫血、抗氧化和增强免疫力、抗癌、医治失眠、美容等保健作用[1-2]。法兰地品种草莓的种苗抗高温、高湿,抗病能力强,果实风味独特、硬度大,适合长途运输,是我国华南地区的草莓主栽品种[3]。为阐明该品种草莓的化学成分,我们从其鲜果的乙醇提取物中分离鉴定了22个酚类、9个降倍半萜类和3个三萜类化合物,并测定了它们的抗氧化和α-葡萄糖苷酶抑制活性[4-5]。本文报道从中分离鉴定的12个芳香类和7个黄酮类化合物。
1 材料和方法 1.1 材料草莓(Fragaria×ananassa Duch.)法兰地品种鲜果于2013年3月采自广州市白云区太和镇草莓种植场。
青岛谱科分离材料公司柱层析硅胶(100~200目);烟台江友硅胶开发公司薄层色谱HSGF254硅胶板;瑞典GE Healthcare Bio-Sciences AB公司葡聚糖凝胶LH-20;美国Amberlite公司大孔树脂XAD-7HP。
1.2 仪器和试剂日本岛津公司LC-6AD高效液相色谱仪和RID-10A视差检测器;利穗科技(苏州)公司EZ Purifier 100中压液相色谱仪;日本东京理化公司旋转蒸发仪;上海沪西分析仪器公司BSZ-100自动部份收集器;美国应用生物系统公司MDS SCIEX API 2000 LC-MS/MS质谱仪测电喷雾质谱(ESI-MS);布鲁克公司Ascend-500核磁共振仪,以Sigma-Aldrich公司氘代甲醇(CD3OD)或氘代二甲基亚砜(DMSO-d6)为溶剂,化学位移(δ, ppm)值以溶剂峰为参照。
提取用乙醇为食用级;石油醚、乙酸乙酯、正丁醇、三氯甲烷、甲醇、甲酸等为分析纯;高效液相色谱用甲醇和乙腈为色谱纯。
1.3 提取和分离将摘除萼片和果柄的草莓鲜果(82.0 kg)切片, 用95%乙醇浸泡3次,每次50 L、2 d,合并滤液, 减压浓缩,得乙醇提取物3.7 kg。提取物用6 L蒸馏水溶解,倒入分液漏斗,加石油醚脱色3次,每次4 L。水液再分别用乙酸乙酯、正丁醇萃取4次,每次4 L,合并萃取液,减压浓缩至干,得乙酸乙酯萃取物68.6 g和正丁醇萃取物360.0 g。将后者过大孔吸附树脂柱,先用2倍柱体积的蒸馏水洗脱,再用4倍柱体积的90%乙醇洗脱,减压浓缩至干,得乙醇洗脱物86.6 g。合并乙酸乙酯萃取物和乙醇洗脱物,进行硅胶柱色谱分离,用三氯甲烷-甲醇混合液[10:0~0:10, (V/V, 下同)]洗脱,收集流份,经薄层色谱检查,合并为7个组分(F1~F7)。F2 (14.8 g)经中压液相色谱分离,用甲醇-水(2:8~10:0)为流动相,流速10 mL min-1,收集流份,经薄层色谱检查,合并为24个亚组分(F2-1~F2-24)。F2-2经葡聚糖凝胶柱色谱分离,用甲醇洗脱,得化合物1 (10 mg)。F2-3经高效液相色谱分离,以乙腈-水-甲酸(10:90:0.1)为流动相,流速5 mL min-1,得化合物4 (保留时间tR=63.1 min, 2 mg)。F2-4经高效液相色谱分离,以乙腈-水-甲酸(20:80:0.1)为流动相,流速5 mL min-1,得化合物2 (tR=60.2 min, 19 mg)。F2-5经中压液相色谱分离,再经高效液相色谱纯化,以乙腈-水-甲酸(20:80:0.1)为流动相,流速5 mL min-1,得化合物8 (tR=72.5 min, 16 mg)、9 (tR=75.1 min, 9 mg)和18 (tR=42.0 min, 13 mg)。F4 (11.0 g)经中压液相色谱分离,用甲醇-水(1:9~8:2)为流动相,流速10 mL min-1,收集流份,经薄层色谱检查,合并为21个亚组分(F4-1~F4-21)。F4-1经葡聚糖凝胶柱色谱分离,用甲醇洗脱,合并为A和B两个亚组分。F4-1A经高效液相色谱纯化,以乙腈-水(10:90)为流动相,流速5 mL min-1,得化合物6 (tR=35.0 min, 10 mg)。F4-1B经高效液相色谱纯化,以乙腈-水(2:8)为流动相,流速5 mL min-1,得化合物10 (tR=100.3 min, 35 mg)、11 (tR= 102.5 min, 15 mg)和12 (tR=72.9 min, 5 mg)。F4-2分别经中压液相色谱和葡聚糖凝胶柱色谱分离,再经高效液相色谱纯化,以乙腈-水(10:90)为流动相,流速5 mL min-1,得化合物5 (tR=87.5 min, 20 mg)。F4-4经葡聚糖凝胶柱色谱分离,再经高效液相色谱纯化,以甲醇-水(30:70)为流动相,流速5 mL min-1,得化合物7 (tR=60.2 min, 17 mg)。F4-8经中压液相色谱分离,得到7个亚组分。F4-8-3经葡聚糖凝胶柱色谱分离,得化合物3 (30 mg)和17 (2 mg)。F4-8-7经葡聚糖凝胶柱色谱分离,得化合物16 (4 mg)。F4-9经硅胶柱色谱分离,得化合物15 (11 mg)。F4-10经葡聚糖凝胶柱色谱分离,合并为7个亚组分。F4-10-4经高效液相色谱纯化,以乙腈-水-甲酸(42:58:0.1)为流动相,流速5 mL min-1,得化合物19 (tR=81.9 min, 27 mg)。F4-11经硅胶柱色谱和葡聚糖凝胶柱色谱分离,得化合物13 (8 mg)。F4-13经硅胶柱色谱分离,得化合物14 (100 mg)。
1.4 结构鉴定化合物1 无色粉末(甲醇);ESI-MS m/z: 137 [M-H]-, 173 [M + Cl]-, 分子量138, 分子式C7H6O3; 1H NMR (CD3OD, 500 MHz): δ 7.88 (2H, d, J = 8.9 Hz, H-2, 6), 6.82 (2H, d, J = 8.9 Hz, H-3, 5); 13C NMR (CD3OD, 125 MHz): δ 122.7 (C-1), 133.0 (C-2, 6), 116.0 (C-3, 5), 163.3 (C-4), 170.1 (C-7)。以上数据与文献[6]报道的一致,故鉴定为对羟基苯甲酸(p-Hydroxybenzoic acid)。
化合物2 无色粉末(甲醇);ESI-MS m/z: 199 [M + H]+, 221 [M + Na]+, 197 [M-H]-, 分子量198, 分子式C9H10O5; 1H NMR (CD3OD, 500 MHz): δ 7.05 (2H, s, H-2, 6), 4.27 (2H, q, J = 7.1 Hz, H2-1′), 1.34 (3H, t, J = 7.1 Hz, H3-2′); 13C NMR (CD3OD, 125 MHz): δ 121.8 (C-1), 110.0 (C-2, 6), 146.4 (C-3, 5), 139.7 (C-4), 168.5 (C-7), 61.7 (C-1′), 14.6 (C-2′)。以上数据与文献[6]报道的一致,故鉴定为没食子酸乙酯(Ethyl gallate)。
化合物3 无色粉末(甲醇);ESI-MS m/z: 447 [M-H]-, 分子量448, 分子式C20H16O12; 1H NMR (DMSO-d6, 500 MHz): δ 7.56 (1H, s, H-5), 7.46 (1H, s, H-5′), 5.52 (1H, d, J = 1.3 Hz, H-1"), 4.06 (1H, dd, J = 3.2, 1.3 Hz, H-2"), 3.79 (1H, dd, J = 9.5, 3.2 Hz, H-3"), 3.32 (1H, dd, J = 9.5, 9.5 Hz, H-4"), 4.15 (1H, dd, J = 9.5, 6.2 Hz, H-5"), 1.07 (3H, d, J = 6.2 Hz, H3-6"); 13C NMR (DMSO-d6, 125 MHz): δ 113.2 (C-1), 111.7 (C-2), 142.4 (C-3), 136.7 (C-4), 152.4 (C-5), 111.3 (C-6), 158.9 (C-7), 112.2 (C-1′), 107.1 (C-2′), 136.2 (C-3′), 140.0 (C-4′), 148.4 (C-5′), 110.3 (C-6′), 158.7 (C-7′), 102.5 (C-1"), 70.2 (C-2"), 70.4 (C-3"), 71.4 (C-4"), 70.6 (C-5"), 17.7 (C-6")。以上数据与文献[7]报道的一致,故鉴定为鞣花酸4-O-α-l-鼠李糖苷(Ellagic acid 4-O-α-l-rhamnoside)。
化合物4 无色粉末(甲醇);ESI-MS m/z: 293 [M + Na]+, 269 [M-H]-, 305 [M + Cl]-, 分子量270, 分子式C13H18O6; 1H NMR (CD3OD, 500 MHz): δ 7.42 (2H, br d, J = 7.2 Hz, H-2, 6), 7.33 (2H, br t, J = 7.2 Hz, H-3, 5), 7.27 (1H, br t, J = 7.2 Hz, H-4), 4.93 (1H, d, J = 11.8 Hz, Ha-7), 4.67 (1H, d, J = 11.8 Hz, Hb-7), 4.36 (1H, d, J = 7.7 Hz, H-1′), 3.90 (1H, dd, J = 11.8, 2.1 Hz, Ha-6′), 3.69 (1H, dd, J = 11.8, 5.5 Hz, Hb-6′); 13C NMR (CD3OD, 125 MHz): δ 139.0 (C-1), 129.2 (C-2, 6), 129.3 (C-3, 5), 128.7 (C-4), 71.7 (C-7), 103.3 (C-1′), 75.1 (C-2′), 78.0 (C-3′), 71.7 (C-4′), 78.1 (C-5′), 62.8 (C-6′)。以上数据与文献[8]报道的一致,故鉴定为苄基β-d-葡萄糖苷(Benzyl β-d-glucoside)。
化合物5 无色粉末(甲醇);ESI-MS m/z: 425 [M + Na]+, 401 [M-H]-, 437 [M + Cl]-, 分子量402, 分子式C18H26O10; 1H NMR (CD3OD, 500 MHz): δ 7.43 (2H, br d, J = 7.2 Hz, H-2, 6), 7.33 (2H, br t, J = 7.2 Hz, H-3, 5), 7.27 (1H, br t, J = 7.2 Hz, H-4), 4.90 (1H, d, J = 11.8 Hz, Ha-7), 4.65 (1H, d, J = 11.8 Hz, Hb-7), 4.33 (1H, d, J = 7.7 Hz, H-1′), 5.05 (1H, d, J = 2.3 Hz, H-1"); 13C NMR (CD3OD, 125 MHz): δ 138.9 (C-1), 129.3 (C-2, 3, 5, 6), 128.7 (C-4), 71.8 (C-7), 103.2 (C-1′), 75.0 (C-2′), 78.3 (C-3′), 71.7 (C-4′), 77.0 (C-5′), 68.7 (C-6′), 110.0 (C-1"), 78.0 (C-2"), 80.6 (C-3"), 75.1 (C-4"), 65.6 (C-5")。以上数据与文献[9]报道的一致,故鉴定为淫羊藿次苷F2 (Icariside F2)。
化合物6 无色粉末(甲醇);ESI-MS m/z: 425 [M + Na]+, 401 [M-H]-, 437 [M + Cl]-, 分子量402, 分子式C18H26O10; 1H NMR (CD3OD, 500 MHz): δ 7.42 (2H, br d, J = 7.2 Hz, H-2, 6), 7.33 (2H, br t, J = 7.2 Hz, H-3, 5), 7.27 (1H, br t, J = 7.3 Hz, H-4), 4.90 (1H, d, J = 11.8 Hz, Ha-7), 4.66 (1H, d, J = 11.8 Hz, Hb-7), 4.36 (1H, d, J = 7.8 Hz, H-1′), 5.00 (1H, br s, H-1"); 13C NMR (CD3OD, 125 MHz): δ 138.9 (C-1), 129.3 (C-2, 3, 5, 6), 128.7 (C-4), 71.9 (C-7), 103.3 (C-1′), 75.1 (C-2′), 78.0 (C-3′), 72.0 (C-4′), 76.8 (C-5′), 68.1 (C-6′), 110.0 (C-1"), 83.3 (C-2"), 78.9 (C-3"), 85.9 (C-4"), 63.1 (C-5")。以上数据与文献[10, 11]报道的相符,故鉴定为苄基6-O-α-l-呋喃阿拉伯糖基-β-d-葡萄糖苷(Benzyl 6-O-α-l-arabinofuranosyl-β-d-glucoside)。
化合物7 白色粉末(甲醇);ESI-MS m/z: 439 [M + Na]+, 415 [M-H]-, 451 [M + Cl]-, 分子量416, 分子式C19H28O10; 1H NMR (CD3OD, 500 MHz): δ 7.23 (4H, m, H-2, 3, 5, 6), 7.15 (1H, m, H-4), 2.92 (1H, t, J = 7.3 Hz, H2-7), 3.26 (1H, m, Ha-8), 3.16 (1H, dd, J = 8.9, 8.0 Hz, Hb-8), 4.29 (1H, d, J = 7.8 Hz, H-1′), 4.94 (1H, d, J = 0.8 Hz, H-1"); 13C NMR (CD3OD, 125 MHz): δ 140.0 (C-1), 130.0 (C-2, 6), 129.4 (C-3, 5), 127.2 (C-4), 37.2 (C-7), 71.9 (C-8), 104.4 (C-1′), 75.1 (C-2′), 78.0 (C-3′), 71.9 (C-4′), 76.7 (C-5′), 68.1 (C-6′), 109.9 (C-1"), 83.2 (C-2"), 78.9 (C-3"), 85.9 (C-4"), 63.1 (C-5")。以上数据与文献[11]报道的一致,故鉴定为苯乙基6-O-α-l-呋喃阿拉伯糖基-β-d-葡萄糖苷(Phenethyl 6-O-α-l-arabinofuranosyl-β-d-glucoside)。
化合物8 白色粉末(甲醇);ESI-MS m/z: 333 [M + Na]+, 309 [M-H]-, 345 [M + Cl]-, 分子量310, 分子式C15H18O7; 1H NMR (CD3OD, 500 MHz): δ 7.63 (2H, dd, J = 6.6, 3.0 Hz, H-2, 6), 7.42 (3H, m, H-3, 4, 5), 7.81 (1H, d, J = 16.0 Hz, H-7), 6.58 (1H, d, J = 16.0 Hz, H-8), 5.60 (1H, d, J = 7.8 Hz, H-1′); 13C NMR (CD3OD, 125 MHz): δ 135.6 (C-1), 130.1 (C-2, 6), 129.4 (C-3, 5), 131.8 (C-4), 147.6 (C-7), 118.3 (C-8), 167.1 (C-9), 95.9 (C-1′), 74.0 (C-2′), 78.0 (C-3′), 71.1 (C-4′), 78.9 (C-5′), 62.3 (C-6′)。以上数据与文献[12]报道的一致,故鉴定为反式肉桂酰基β-d-葡萄糖苷(trans-Cinnamoyl β-d-glucoside)。
化合物9 白色粉末(甲醇);ESI-MS m/z: 333 [M + Na]+, 309 [M-H]-, 345 [M + Cl]-, 分子量310, 分子式C15H18O7; 1H NMR (CD3OD, 500 MHz): δ 7.68 (2H, dd, J = 6.5, 2.8 Hz, H-2, 6), 7.35 (3H, m, H-3, 4, 5), 7.08 (1H, d, J = 12.7 Hz, H-7), 6.02 (1H, d, J = 12.7 Hz, H-8), 5.54 (1H, d, J = 8.2 Hz, H-1′); 13C NMR (CD3OD, 125 MHz): δ 135.9 (C-1), 131.3 (C-2, 6), 129.0 (C-3, 5), 130.4 (C-4), 146.5 (C-7), 119.4 (C-8), 166.1 (C-9), 95.7 (C-1′), 73.9 (C-2′), 78.1 (C-3′), 71.1 (C-4′), 78.9 (C-5′), 62.4 (C-6′)。以上数据与文献[12]报道的一致,故鉴定为顺式肉桂酰基β-d-葡萄糖苷(cis-Cinnamoyl β-d-glucoside)。
化合物10 白色粉末(甲醇);ESI-MS m/z: 349 [M + Na]+, 325 [M-H]-, 361 [M + Cl]-, 分子量326, 分子式C15H18O8; 1H NMR (DMSO-d6, 500 MHz): δ 7.58 (2H, d, J = 8.6 Hz, H-2, 6), 6.80 (2H, d, J = 8.6 Hz, H-3, 5), 7.64 (1H, d, J = 15.9 Hz, H-7), 6.40 (1H, d, J = 15.9 Hz, H-8), 5.46 (1H, d, J = 8.1 Hz, H-1′); 13C NMR (DMSO-d6, 125 MHz): δ 125.0 (C-1), 130.5 (C-2, 6), 115.8 (C-3, 5), 160.1 (C-4), 146.0 (C-7), 113.6 (C-8), 165.4 (C-9), 94.2 (C-1′), 72.5 (C-2′), 76.5 (C-3′), 69.5 (C-4′), 77.8 (C-5′), 60.6 (C-6′)。以上数据与文献[13]报道的一致,故鉴定为反式对香豆酰基β-d-葡萄糖苷(trans-p-Coumaroyl β-d-glucoside)。
化合物11 白色粉末(甲醇);ESI-MS m/z: 349 [M + Na]+, 325 [M-H]-, 361 [M + Cl]-, 分子量326, 分子式C15H18O8; 1H NMR (DMSO-d6, 500 MHz): δ 7.74 (2H, d, J = 8.6 Hz, H-2, 6), 6.77 (2H, d, J = 8.6 Hz, H-3, 5), 6.96 (1H, d, J = 12.9 Hz, H-7), 5.78 (1H, d, J = 12.9 Hz, H-8), 5.43 (1H, d, J = 8.1 Hz, H-1′); 13C NMR (DMSO-d6, 125 MHz): δ 125.2 (C-1), 133.1 (C-2, 6), 114.9 (C-3, 5), 159.2 (C-4), 145.3 (C-7), 114.4 (C-8), 164.5 (C-9), 94.1 (C-1′), 72.4 (C-2′), 76.5 (C-3′), 69.5 (C-4′), 77.9 (C-5′), 60.6 (C-6′)。由H-7与H-8的耦合常数12.9 Hz可判断苷元中的双键为顺式构型[14],故鉴定为顺式对香豆酰基β-d-葡萄糖苷(cis-p-Coumaroyl β-d-glucoside)。
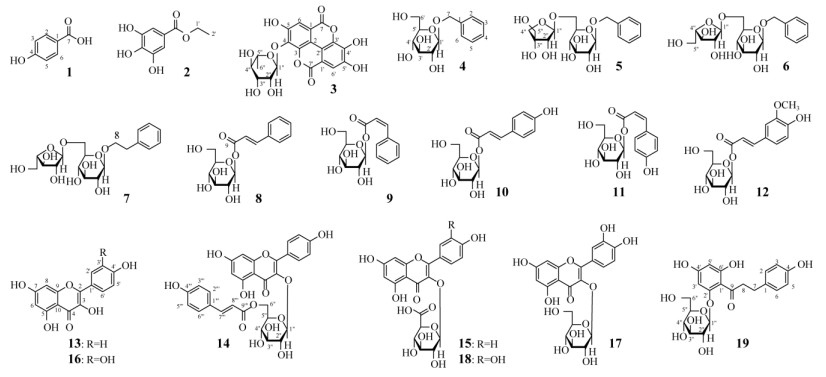
|
图 1 化合物1~19的结构 Fig. 1 Structures of compounds 1–19 |
化合物12 白色粉末(甲醇);ESI-MS m/z: 355 [M-H]-, 391 [M + Cl]-, 分子量356, 分子式C16H20O9; 1H NMR (DMSO-d6, 500 MHz): δ 7.33 (1H, d, J = 1.5 Hz, H-2), 6.80 (1H, d, J = 8.2 Hz, H-5), 7.15 (1H, dd, J = 8.2, 1.5 Hz, H-6), 7.63 (1H, d, J = 15.9 Hz, H-7), 6.48 (1H, d, J = 15.9 Hz, H-8), 3.81 (3H, s, OCH3), 5.46 (1H, d, J = 8.0 Hz, H-1′); 13C NMR (DMSO-d6, 125 MHz): δ 125.4 (C-1), 115.6 (C-2), 148.0 (C-3), 149.8 (C-4), 111.4 (C-5), 123.4 (C-6), 146.4 (C-7), 113.9 (C-8), 165.5 (C-9), 56.7 (OCH3), 94.3 (C-1′), 72.6 (C-2′), 77.9 (C-3′), 69.6 (C-4′), 76.6 (C-5′), 60.7 (C-6′)。以上数据与文献[15](测定溶剂为CD3OD)报道的基本一致,故鉴定为反式阿魏酰基β-d-葡萄糖苷(trans-Feruloyl β-d-glucoside)。
化合物13 黄色粉末(甲醇);ESI-MS m/z: 285 [M-H]-, 分子量286, 分子式C15H10O6; 1H NMR (CD3OD, 500 MHz): δ 6.20 (1H, d, J = 2.1 Hz, H-6), 6.39 (1H, d, J = 2.1 Hz, H-8), 8.08 (2H, d, J = 8.9 Hz, H-2′, 6′), 6.90 (2H, d, J = 8.9 Hz, H-3′, 5′); 13C NMR (CD3OD, 125 MHz): δ 148.0 (C-2), 137.1 (C-3), 177.4 (C-4), 162.5 (C-5), 99.3 (C-6), 165.6 (C-7), 94.5 (C-8), 158.2 (C-9), 104.5 (C-10), 123.7 (C-1′), 130.7 (C-2′, 6′), 116.3 (C-3′, 5′), 160.6 (C-4′)。以上数据与文献[6]报道的一致,故鉴定为山柰酚(Kaempferol)。
化合物14 黄色粉末(甲醇);ESI-MS m/z: 617 [M + Na]+, 593 [M-H]-, 629 [M + Cl]-, 分子量594, 分子式C30H26O13; 1H NMR (DMSO-d6, 500 MHz): δ 12.58 (1H, 5-OH), 6.15 (1H, d, J = 2.1 Hz, H-6), 6.39 (1H, d, J = 2.1 Hz, H-8), 7.99 (2H, d, J = 8.8 Hz, H-2′, 6′), 6.86 (2H, d, J = 8.8 Hz, H-3′, 5′), 5.45 (1H, d, J = 7.3 Hz, H-1"), 4.27 (1H, dd, J = 12.0, 2.2 Hz, Ha-6"), 4.03 (1H, dd, J = 12.0, 6.3 Hz, Hb-6"), 7.37 (2H, d, J = 8.4 Hz, H-2''', 6'''), 6.81 (2H, d, J = 8.4 Hz, H-3''', 5'''), 7.34 (1H, d, J = 15.9 Hz, H-7'''), 6.12 (1H, d, J = 15.9 Hz, H-8'''); 13C NMR (DMSO-d6, 125 MHz): δ 156.2 (C-2), 136.4 (C-3), 177.4 (C-4), 161.1 (C-5), 98.7 (C-6), 164.1 (C-7), 93.6 (C-8), 156.4 (C-9), 103.9 (C-10), 120.7 (C-1′), 130.8 (C-2′, 6′), 115.7 (C-3′, 5′), 160.0 (C-4′), 101.0 (C-1"), 74.1 (C-2"), 76.2 (C-3"), 69.9 (C-4"), 74.2 (C-5"), 63.0 (C-6"), 124.9 (C-1'''), 130.1 (C-2''', 6'''), 115.1 (C-3''', 5'''), 159.8 (C-4'''), 144.6 (C-7'''), 113.6 (C-8'''), 166.1 (C-9''')。以上数据与文献[16]报道的一致,故鉴定为山柰酚3-O-(6-O-反式对香豆酰基)-β-d-葡萄糖苷[Kaempferol 3-O-(6-O-trans-p-coumaroyl)-β-d-glucoside],即反式椴树苷(trans-Tiliroside)。
化合物15 黄色粉末(甲醇);ESI-MS m/z: 463 [M + H]+, 485 [M + Na]+, 461 [M-H]-, 分子量462, 分子式C21H18O12; 1H NMR (DMSO-d6, 500 MHz): δ 12.53 (1H, s, 5-OH), 6.21 (1H, d, J = 2.0 Hz, H-6), 6.43 (1H, d, J = 2.0 Hz, H-8), 8.04 (2H, d, J = 8.8 Hz, H-2′, 6′), 6.87 (2H, d, J = 8.8 Hz, H-3′, 5′), 5.49 (1H, d, J = 7.1 Hz, H-1"); 13C NMR (DMSO-d6, 125 MHz): δ 156.3 (C-2), 133.0 (C-3), 177.2 (C-4), 160.1 (C-5), 98.8 (C-6), 164.3 (C-7), 93.7 (C-8), 161.2 (C-9), 103.9 (C-10), 120.6 (C-1′), 130.9 (C-2′, 6′), 115.1 (C-3′, 5′), 156.3 (C-4′), 101.1 (C-1"), 73.9 (C-2"), 75.8 (C-3"), 71.5 (C-4"), 75.8 (C-5"), 169.9 (C-6")。以上数据与文献[17]报道的一致,故鉴定为山柰酚3-O-β-d-葡萄糖醛酸苷(Kaempferol 3-O-β-d-glucuronide)。
化合物16 黄色粉末(甲醇);ESI-MS m/z: 325 [M + Na]+, 301 [M-H]-, 分子量302, 分子式C15H10O7; 1H NMR (CD3OD, 500 MHz): δ 6.18 (1H, d, J = 2.1 Hz, H-6), 6.39 (1H, d, J = 2.1 Hz, H-8), 7.74 (1H, d, J = 2.2 Hz, H-2′), 8.09 (1H, d, J = 8.6 Hz, H-5′), 7.64 (1H, dd, J = 8.6, 2.2 Hz, H-6′); 13C NMR (CD3OD, 125 MHz): δ 158.3 (C-2), 130.7 (C-3), 177.3 (C-4), 162.5 (C-5), 99.2 (C-6), 165.6 (C-7), 94.4 (C-8), 158.2 (C-9), 104.5 (C-10), 124.1 (C-1′), 116.3 (C-2′), 148.0 (C-3′), 148.8 (C-4′), 116.0 (C-5′), 121.7 (C-6′)。以上数据与文献[6]报道的一致,故鉴定为槲皮素(Quercetin)。
化合物17 黄色粉末(甲醇);ESI-MS m/z: 487 [M + Na]+, 463 [M-H]-, 分子量464, 分子式C21H20O12; 1H NMR (CD3OD, 500 MHz): δ 6.18 (1H, d, J = 2.0 Hz, H-6), 6.37 (1H, d, J = 2.1 Hz, H-8), 7.71 (1H, d, J = 2.2 Hz, H-2′), 6.87 (1H, d, J = 8.5 Hz, H-5′), 7.59 (1H, dd, J = 8.5, 2.2 Hz, H-6′), 5.23 (1H, d, J = 7.7 Hz, H-1"), 3.71 (1H, dd, J = 11.8, 2.2 Hz, Ha-6"), 3.58 (1H, dd, J = 11.8, 5.4 Hz, Hb-6"); 13C NMR (CD3OD, 125 MHz): δ 159.2 (C-2), 135.6 (C-3), 179.5 (C-4), 163.1 (C-5), 100.0 (C-6), 166.1 (C-7), 94.9 (C-8), 158.5 (C-9), 105.9 (C-10), 123.2 (C-1′), 117.2 (C-2′), 146.0 (C-3′), 149.9 (C-4′), 116.0 (C-5′), 123.2 (C-6′), 104.4 (C-1"), 75.7 (C-2"), 78.1 (C-3"), 71.2 (C-4"), 78.4 (C-5"), 62.5 (C-6")。以上数据与文献[18]报道的一致,故鉴定为槲皮素3-O-β-d-葡萄糖苷(Quercetin 3-O-β-d-glucoside)。
化合物18 黄色粉末(甲醇);ESI-MS m/z: 479 [M + H]+, 501 [M + Na]+, 477 [M-H]-, 分子量478, 分子式C21H18O13; 1H NMR (DMSO-d6, 500 MHz): δ 12.45 (1H, br s, 5-OH), 6.20 (1H, br s, H-6), 6.40 (1H, br s, H-8), 7.84 (1H, br s, H-2′), 6.83 (1H, d, J = 7.0 Hz, H-5′), 7.50 (1H, d, J = 7.0 Hz, H-6′), 5.39 (1H, d, J = 6.3 Hz, H-1"); 13C NMR (DMSO-d6, 125 MHz): δ 156.8 (C-2), 133.5 (C-3), 177.3 (C-4), 161.1 (C-5), 98.8 (C-6), 164.4 (C-7), 93.6 (C-8), 156.3 (C-9), 103.8 (C-10), 121.2 (C-1′), 116.9 (C-2′), 144.9 (C-3′), 148.5 (C-4′), 115.3 (C-5′), 120.7 (C-6′), 101.8 (C-1"), 73.9 (C-2"), 76.2 (C-3"), 71.6 (C-4"), 75.2 (C-5"), 170.9 (C-6")。以上数据与文献[17]报道的一致,故鉴定为槲皮素3-O-β-d-葡萄糖醛酸苷(Quercetin 3-O-β-d-glucuronide)。
化合物19 黄色粉末(甲醇);ESI-MS m/z: 459 [M + Na]+, 435 [M-H]-, 471 [M + Cl]-, 分子量436, 分子式C21H24O10; 1H NMR (CD3OD, 500 MHz): δ 7.06 (2H, d, J = 8.2 Hz, H-2, 6), 6.69 (2H, d, J = 8.2 Hz, H-3, 5), 2.88 (2H, t, J = 7.5 Hz, H2-7), 6.19 (1H, br s, H-3′), 5.96 (1H, br s, H-5′), 5.04 (1H, d, J = 6.3 Hz, H-1"), 3.91 (1H, br d, J = 11.9 Hz, Ha-6"), 3.72 (1H, dd, J = 11.8, 4.5 Hz, Hb-6"); 13C NMR (CD3OD, 125 MHz): δ 133.9 (C-1), 130.4 (C-2, 6), 116.1 (C-3, 5), 156.3 (C-4), 30.8 (C-7), 46.9 (C-8), 206.5 (C-9), 106.8 (C-1′), 167.5 (C-2′), 95.4 (C-3′), 165.8 (C-4′), 98.3 (C-5′), 162.3 (C-6′), 102.0 (C-1"), 74.7 (C-2"), 78.4 (C-3"), 71.1 (C-4"), 78.4 (C-5"), 62.4 (C-6")。以上数据与文献[19]报道的一致,故鉴定为根皮苷(Phloridzin)。
2 结果和讨论采用色谱分离方法,从草莓法兰地品种鲜果中分离得到19个化合物,分别鉴定为对羟基苯甲酸(1)、没食子酸乙酯(2)、鞣花酸3-O-α-l-鼠李糖苷(3)、苄基β-d-葡萄糖苷(4)、淫羊藿次苷F2 (5)、苄基6-O-α-l-呋喃阿拉伯糖基-β-d-葡萄糖苷(6)、苯乙基6-O-α-l-呋喃阿拉伯糖基-β-d-葡萄糖苷(7)、反式肉桂酰基β-d-葡萄糖苷(8)、顺式肉桂酰基β-d-葡萄糖苷(9)、反式对香豆酰基β-d-葡萄糖苷(10)、顺式对香豆酰基β-d-葡萄糖苷(11)、反式阿魏酸酰基β-d-葡萄糖苷(12)、山柰酚(13)、反式椴树苷(14)、山柰酚3-O-β-d-葡萄糖醛酸苷(15)、槲皮素(16)、槲皮素3-O-β-d-葡萄糖苷(17)、槲皮素3-O-β-d-葡萄糖醛酸苷(18)和根皮苷(19)。所有化合物均为首次从该品种草莓中报道,其中化合物4~9为首次从草莓中报道。
化合物1~3和10~19均含酚羟基,具有清除自由基和抑制α-葡萄糖苷酶活性[4]。对羟基苯甲酸有防腐、防霉和杀菌作用。没食子酸乙酯在体外能降低高转移性人乳腺癌MDA-MB-231的侵袭能力,可能与抑制癌细胞中原癌基因和延长因子蛋白以及mRNA的表达有关[20]。鞣花酸及其糖苷具有抗癌、抗突变、抑制人体免疫缺陷病毒、凝血等作用,能保护创面免受细菌的侵入,防止感染、抑制溃疡,还具有降压、镇痛作用[21]。淫羊藿次苷F2能抑制肿瘤坏死因子α (TNF-α)刺激的核因子κB (NF-κB)的产生(IC50=16.25 μmol L–1),显示出抗炎活性[22]。山柰酚具有防癌、抗癌、抗感染、抗炎作用,对动脉粥样硬化和糖尿病的防治作用,对蛋白激酶的抑制和免疫抑制作用,还能对抗β-淀粉样蛋白对神经元的损伤等[23]。反式银椴苷能有效地抑制糖负荷小鼠的体重增加,尤其是内脏脂肪重量的增加及血浆中葡萄糖水平的增加[24]。槲皮素具有抗肿瘤、抗氧化、抗感染(对金黄色葡萄球菌的最低杀菌浓度小于6.1 μmol L–1)、免疫抑制、心血管保护和血糖调节等多种药理作用[25]。槲皮素3-葡萄糖苷可明显改善大鼠脑缺血-再灌注损伤,减轻缺血再灌注导致的脑水肿、氧化损伤和能量代谢障碍,对心肌缺血及再灌注损伤也有明显的保护作用[26]。根皮苷能降低糖尿病小鼠的血糖、甘油三酯和总胆固醇含量;对于高膳食导致的氧化损伤的果蝇具有明显的保护作用,其抗氧化活性强于维生素E;还具有抗炎、抗癌作用[27]。
| [1] |
LUO X B, HE L M. Nutritional value and health function of strawberry[J].
Food Nutri China, 2011, 17(4): 74-76. 罗学兵, 贺良明. 草莓的营养价值与保健功能[J]. 中国食物与营养, 2011, 17(4): 74-76. DOI:10.3969/j.issn.1006-9577.2011.04.020 |
| [2] |
CHENG R, SHENG J P. Research progress on polyphenolic phyto-chemicals of strawberry fruits[J].
J Food Saf Qual, 2015, 6(2): 575-584. 程然, 生吉萍. 草莓多酚类植物化学物研究进展[J]. 食品安全质量检测学报, 2015, 6(2): 575-584. |
| [3] |
LIU Y J, PENG L T, YE J L, et al. Components and stabilities of anthocyanins from 'Faland' strawberry fruits[J].
J Huazhong Agric Univ, 2016, 31(1): 24-30. 刘雨佳, 彭丽桃, 叶俊丽, 等. '法兰地'草莓果实中花色素苷的组成及稳定性[J]. 华中农业大学学报, 2016, 31(1): 24-30. |
| [4] | YANG D, XIE H H, JIANG Y M, et al. Phenolics from strawberry cv. Falandi and their antioxidant and α-glucosidase inhibitory activities[J]. Food Chem, 2016, 194: 857-863. DOI:10.1016/j.foodchem.2015.08.191 |
| [5] | YANG D, LIANG J, XIE H H, et al. Norsesquiterpenoids and triterpenoids from strawberry cv. Falandi[J]. Food Chem, 2016, 203(1): 67-72. DOI:10.1016/j.foodchem.2016.02.036 |
| [6] |
WANG H P, CAO F, YANG X W. Chemical constituents in aerial parts of Polygonum capitatum[J].
Chin Trad Herb Med, 2013, 44(1): 24-30. 王洪平, 曹芳, 杨秀伟. 头花蓼地上部分的化学成分研究[J]. 中草药, 2013, 44(1): 24-30. DOI:10.7501/j.issn.0253-2670.2013.01.006 |
| [7] | LI L, ZHAO Y Y, LIU W Y, et al. HPLC with quadrupole TOF-MS and chemometrics analysis for the characterization of Folium turpiniae from different regions[J]. J Sep Sci, 2013, 36(15): 2552-2561. DOI:10.1002/jssc.201300360 |
| [8] | de MARINO S, FESTA C, ZOLLO F, et al. Phenolic glycosides from Cucumis melo var. inodorus seeds[J]. Phytochem Lett, 2009, 2(3): 130-133. DOI:10.1016/j.phytol.2009.04.001 |
| [9] | WANG M F, LI J G, RANGARAJAN M, et al. Antioxidative phenolic compounds from sage (Salvia officinalis)[J]. J Agric Food Chem, 1998, 46(12): 4869-4873. DOI:10.1021/jf980614b |
| [10] |
YANG H L, YU S S, PEI Y H. Studies on chemical constituents from leaves of Lysidice brevicalyx[J].
China J Chin Mat Med, 2008, 33(22): 2633-2635. 杨华良, 虞石山, 裴月湖. 短萼仪花叶化学成分研究[J]. 中国中药杂志, 2008, 33(22): 2633-2635. DOI:10.3321/j.issn:1001-5302.2008.22.014 |
| [11] | MA S J, MIZUTANI M, HIRATAKE J, et al. Substrate specificity of β-primeverosidase, a key enzyme in aroma formation during oolong tea and black tea manufacturing[J]. Biosci Biotechnol Biochem, 2001, 65(12): 2719-2729. DOI:10.1271/bbb.65.2719 |
| [12] | HIRADATE S, MORITA S, SUGIE H, et al. Phytotoxic cis-cinnamoyl glucosides from Spiraea thunbergii[J]. Phytochemistry, 2004, 65(6): 731-739. DOI:10.1016/j.phytochem.2004.01.010 |
| [13] | INA H, KOMAKID K, ⅡDA H. Hydroxycinnamylglucoses from Spiraea thunbergii[J]. Planta Med, 1987, 53(5): 502 DOI:10.1055/s-2006-962788 |
| [14] |
CHEN Q, WANG T, GE D D, et al. Isolation and identification of flavonoids from Leontopodium leontopodioides (Willd.) Beauv. (Ⅱ)[J].
J Shenyang Pharm Univ, 2012, 29(2): 104-108. 陈秋, 王涛, 葛丹丹, 等. 火绒草黄酮类成分的分离与鉴定(Ⅱ)[J]. 沈阳药科大学学报, 2012, 29(2): 104-108. |
| [15] | BADERSCHNEIDER B, WINTERHALTER P. Isolation and chara-cterization of novel benzoates, cinnamates, flavonoids, and lignans from riesing wine and screening for antioxidant activity[J]. J Agric Food Chem, 2001, 49(6): 2788-2798. DOI:10.1021/jf010396d |
| [16] |
SUN Q, WU J, LI F F, et al. Isolation and identification of chemical constituents from Daphne genkwa Sieb. et Zucc.[J].
J Shenyang Pharm Univ, 2014, 31(2): 94-98. 孙倩, 武洁, 李菲菲, 等. 芫花化学成分的分离与鉴定[J]. 沈阳药科大学学报, 2014, 31(2): 94-98. |
| [17] |
DUAN Y H, DAI Y, GAO H, et al. Chemical constituents from Sarcandra glabra[J].
Chin Trad Herb Med, 2010, 41(1): 29-32. 段营辉, 戴毅, 高昊, 等. 草珊瑚的化学成分研究[J]. 中草药, 2010, 41(1): 29-32. DOI:10.7501/j.issn.0253-2670.2010.1.009 |
| [18] | FERNANDEZ J, REYS R, PONCE H, et al. Isoquercitrin from Argemone platyceras inhibits carbachol and leukotriene D4-induced contraction in guinea-pig airways[J]. Eurp J Pharmacol, 2005, 522(1/2/3): 108-115. DOI:10.1016/j.ejphar.2005.08.046 |
| [19] | CUENDET M, POTTERAT O, SALVI A, et al. A stilbene and dihydrochalcones with radical scavenging activities from Loiseleuria procumbens[J]. Phytochemistry, 2000, 54(8): 871-874. DOI:10.1016/S0031-9422(00)00200-4 |
| [20] |
HUANG L Y, CHEN X. The effect of ethyl gallate on the invasion ability of human breast cancer MDA-MB-231 cells and the underlying mechanism of action[J].
Oncol Progr, 2016, 14(4): 375-377. 黄丽英, 陈夏. 没食子酸乙酯对人乳腺癌MDA-MB-231细胞侵袭能力及其作用机制研究[J]. 癌症进展, 2016, 14(4): 375-377. DOI:10.11877/j.issn.1672-1535.2016.14.04.24 |
| [21] |
DING Y S, SUN X H, LI Y G, et al. Recent progress of ellagic acid and its derivatives[J].
J Hefei Univ Technol, 2008, 31(11): 1809-1812. 丁运生, 孙小虎, 李有桂, 等. 鞣花酸及其衍生物研究进展[J]. 合肥工业大学学报, 2008, 31(11): 1809-1812. DOI:10.3969/j.issn.1003-5060.2008.11.020 |
| [22] | BAI M M, SHI W, TIAN J M, et al. Soluble epoxide hydrolase inhibitory and anti-inflammatory components from the leaves of Eucommia ulmoides Oliver (Duzhong)[J]. J Agric Food Chem, 2015, 63(8): 2198-2205. DOI:10.1021/acs.jafc.5b00055 |
| [23] |
CHEN Y H, ZHOU K Y, YUAN H Y. Research progress on kaempferol pharmacodynamics[J].
Guangdong Med J, 2010, 31(8): 1064-1066. 陈育华, 周克元, 袁汉尧. 山奈酚药效的研究进展[J]. 广东医学, 2010, 31(8): 1064-1066. DOI:10.3969/j.issn.1001-9448.2010.08.058 |
| [24] | NINOMIYA K, MATSUDA H, KUBO M, et al. Potent anti-obese principle from Rosa canina:Structural requirements and mode of action of trans-tiliroside[J]. Bioorg Med Chem Lett, 2007, 17(11): 3059-3064. DOI:10.1016/j.bmcl.2007.03.051 |
| [25] |
LUO M X, LUO D, ZHAO W H. Research progress on pharmaco-logical action of quercetin[J].
Chin J Enthnomed Enthnopharm, 2014, 17(1): 12-14. 骆明旭, 罗丹, 赵万红. 槲皮素药理作用研究进展[J]. 中国民族民间医药, 2014, 17(1): 12-14. |
| [26] |
HU Q R, JIA Z. Protective effects of quercetin 3-glucoside on cerebral ischemia-reperfusion injury in rats[J].
Clin J Chin Med, 2015, 7(3): 33-35. 胡清茹, 贾真. 槲皮素-3-葡萄糖苷对大鼠脑缺血-再灌注损伤的保护作用[J]. 中医临床研究, 2015, 7(3): 33-35. DOI:10.3969/j.issn.1674-7860.2015.03.015 |
| [27] |
TAN S, ZHOU Z Q. Current research status on phloridzin[J].
Food Ferm Ind, 2013, 39(8): 182-186. 谭飔, 周志钦. 根皮苷研究进展[J]. 食品与发酵工业, 2013, 39(8): 182-186. DOI:10.13995/j.cnki.11-1802/ts.2013.08.004 |
 2017, Vol. 25
2017, Vol. 25


