Camellia sinensis and C. oleifera, belonging to Theaceae, are important economic plants in China. Currently, tea made from C. sinensis is one of the most popular beverages together with coffee and cocoa all over the world. Phenolic compounds, theanine and purine alkaloids are the main specific and important secondary metabolites related to tea quality and flavor in tea liquor. Recent studies have confirmed that the beneficial health effects, such as reducing blood lipid, anti-cancer and antioxidant activity, could be all attributed to these compounds[1]. Furthermore, the biosynthesis and degradation of these metabolites were also reported. Purine alkaloids derived from purine nucleotides, mainly including caffeine, 7-methylxanthine, theophylline and theobromine in tea[2-3]. Among amino acids, theanine, a non-protein amino acid found in tea leaves by Sakato[4], has a special taste described as "umami"[5]. And phenolic compounds are important secondary metabolites in plants. Among phenolic compounds in tea leaves, non-gallate catechins, EC (epicatechin), and EGC (epigallocatechin); gallate catechins, ECG (epicatechin gallate) and EGCG (epigallocatechin gallate) are specific phenolic compounds related to tea quality.
Camellia oleifera, whose fruits and oil have high nutritional and medicinal values, is a unique woody edible oil tree in China, and is one of the four major woody oil crops in the world[6]. Oil made from seeds of C. oleifera could be also called 'eastern olive oil' at present[7]. The contents of benzene-ethanol extracts, holo-cellulose, cellulose, lignin and other chemical compositions in shoots and leaves of three ecotypes of C. oleifera had been determined[8]. It is the fact that the cultivation history and application of C. oleifera was long in our country, but researches on the biosynthesis and degradation of some specific metabolites were still undefined[6].
Grafting involves interspecific and heteroplastic graftings. Interspecific grafting among the same Camellia has been completed, and the content of theanine in grafted plants enhanced compared with corresponding scion[9]. Grafting technique has been used to improve the characteristics of C. sinensis and select strains for enhancing some special resistance[10]. Similarly, interspecific grafting has also been used to select better varieties ofC. oleifera because of low yield. As the requirements of production, the method of bud or wood grafting onto selected roots has been popularized[11]. However, heteroplastic grafting between C. sinensis and C. oleifera was rarely carried out at present. Here, we reported a grafted seedlings with C. sinensis as scions and C. oleifera as stocks, the morphological anatomy and biochemical components analysis were studied, which would produce positive impact on the cultivation of C. sinensis and C. oleifera, and play positive roles in the biosynthesis and transportation mechanisms of secondary metabolites in tea plants.
1 Materials and methods 1.1 MaterialsThe grafted seedlings with Camellia sinensis 'Shuchazao' as scions and C. oleifera as stocks were cultivated in Shucheng, Liu'an City, Anhui Province, China. Fresh leaves (one bud and two leaves from the top) were collected from one-year-old grafted seedlings, C. sinensis seedlings and C. oleifera seedlings, frozen directly in liquid nitrogen and stored at-80℃ until use for HPLC determination. For the morphological anatomy analysis, fresh leaves were used. Three replications were carried out individually.
1.2 Morphological anatomy observationThe leaves were immediately dehydrated in FAA mixture and embedded in paraffin mixture gradually[12]. Thereafter, the leaves were cross-sectioned into slices with 12 μm thickness using rotary microtome (Ernst Leitz Wetzlar Gmbh 530586258, Germany). The structure of leaves was observed at 100× magnification under light microscope (Nikon, Eclipse, E200, Japan), and three images were photoed randomly from every section by using Sharp Capture. Meanwhile all morphological indexes were measured three times in every image using Super Image 6.0.1.2.
1.3 Sample extractionFrozen leaves were grinded in liquid nitrogen. The grinded powders were transferred into eppendorf tubes, kept in a deep freezer for 24 h, and then dried by freeze dryer for 48 h. Freeze-dried powder 0.15 g were dissolved in 3 mL 80% (V/V) methanol and extracted by ultrasonic sonicator for 10 min at room temperature. After centrifugation at 3000×g for 10 min, the residues were re-extracted twice as above. The merged supernatants were filtered through a 0.22 μm organic membrane. The extraction of amino acid was performed according to above method with slight modification. The extraction reagent was changed to deionized water instead of 80% methanol and extracted with water bath for 20 min at 100℃.
1.4 HPLC analysisPurine alkaloids were detected using Waters e2695 equipped with 2489 ultraviolet (UV)-visible detector. A reverse-phase C18 column (Gemini 5 μm, 250 mm×4.60 mm, Phenomenex, USA) was used at flow rate of 1.0 mL min-1. The detection wavelength was 274 nm, the column oven temperature was 25℃. The mobile phase composition was according to the method described by Xu, et al[13]with a slight modification as follows: 0.2% (V/V) acetic acid (A) and 100% (V/V) acetonitrile (B), and the gradient conditions as follows: A 92% at 0 min, 83% (V/V) at 25 min, 15% at 30 min, 10% at 32 min, 92% at 35 min, 92% at 40 min. HPLC grade acetic acid, methanol and acetonitrile were obtained from Tedia Co., Ltd. (Fairfield, OH, USA). Caffeine, 7-methylxanthine and theobromine standards were obtained from Sangon Biotech (Shanghai) Co., Ltd.
Amino acids in leaves were detected using a Waters 600E series HPLC equipped with a quaternary pump, a 2475 fluorescence detector, and a 2489 ultraviolet (UV)-visible detector, fluorescence detector, λex=250 nm, λem=395 nm; column temperature, 37℃; injection volume, 5 μL. A Waters AccQ·Tag reverse-phase C18 column was used at flow rate of 1.0 mL min-1. The detection wavelength was 199 nm for analysis. The conditions of HPLC were according to the method described by Xu et al[13]with a slight modification. The mobile phase consisted of 9% (V/V) AccQ·Tag eluent as solvent A, 100% (V/V) acetonitrile as solvent B, and deionized water as solvent C. The gradient conditions were as follows: 0 min, 100% A; 17 min, 91% A, 5% B; 24 min, 80% A, 17% B; 32 min, 68% A, 20% B; 34 min, 68% A, 20% B; 35 min, 0% A, 40% B; 37 min, 0% A, 60% B; 38 min, 100% A; to 45 min, 100% A. Flow rate was 1.0 mL min-1. Amino Acid Standards (AAS18-5ML) were purchased from Sigma-Aldrich Co., Ltd., theanine standard from Shanghai Yuanye Biological Technology Co., Ltd., and AccQ·Tag Kit from Waters (Milford, MA, USA).
Phenolic compounds were detected by Waters 2695 equipped with 2489 ultraviolet (UV)-visible detector. A reverse-phase C18 column (Gemini 5 μm, 250 mm×4.60 mm, Phenomenex, USA) was used at flow rate of 1.0 mL min-1. The detection wavelength was 278 nm and the column oven temperature was 25℃. The mobile phase composition was according to the method described by Jiang et al[14] with slight modification as follows: 0.17% (V/V) acetic acid (A) and 100% (V/V) acetonitrile (B), and the latter gradient as follows: B 6% for 0 min, 14% (V/V) for 16 min, 15% for 22 min, 18% for 32 min, 89% for 37 min, 45% for 45 min, 45% for 50 min, 6% for 51 min, and 6% for 60 min. The standards of C, EC, GC, EGC, EGCG, ECG, βG, GA, Gd were purchased from Sigma (St Louis, MO, USA).
1.5 Statistical analysisAll data were showed as mean±standard deviation. Analysis of variance was performed with Data Processing System (DPS). Statistical analyses were performed with SIMCA (Version 13.0.3).
2 Results 2.1 Principle components and anatomic structure of leavesThe morphology characteristics of transection include thickness of leaf (A), thickness of upper epidermis (B), thickness of upper cuticle (C), thickness of palisade tissue (D), thickness of spongy tissue (E), thickness of lower epidermis (F), thickness of lower cuticle (G), thickness of midrib (H), ratio of palisade tissue to spongy tissue (I), fineness of leaf tissue (J), porosity of leaf tissue (K) and midrib protuberant degree (L). The transections of C. sinensis, C. oleifera and grafted plant leaf were composed of upper and lower epidermises, upper and lower cuticle, palisade tissue, and spongy tissue. The principle component analysis (PCA) showed that the contribution ratio of indexes A to G, I, and J were higher in PCA (Fig. 1). They are the most important factors affecting cluster pattern via PCA (Fig. 2). The cluster pattern showed the relationships among C. sinensis, C. oleifera and grafted leaves. Apparently, C. sinensis, C. oleifera and grafted plants clearly separated from each other, forming distinct cluster. C. sinensis and grafted plants clustered together were independent from C. oleifera. These indicated that the characters of upper part of grafted seedlings mainly closed to their corresponding scions. There were significant differences (P < 0.05) in these indexes among three taxa.
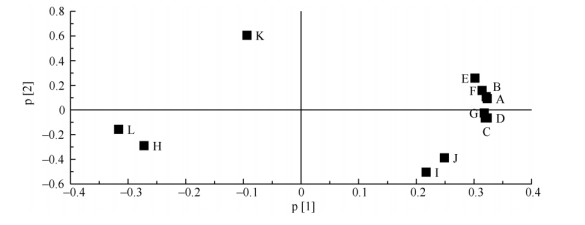
|
Fig. 1 Contribution ratio of leaf morphological indexes by principle component analysis (PCA). A: Thickness of leaf; B: Thickness of upper epidermis; C: Thickness of upper cuticle; D: Thickness of palisade tissue; E: Thickness of spongy tissue; F: Thickness of lower epidermis; G: Thickness of lower cuticle; H: Thickness of midrib; I: Ratio of palisade tissue to spongy tissue; J: Fineness of leaf tissue; K: Porosity of leaf tissue; L: Midrib protuberant degree. |
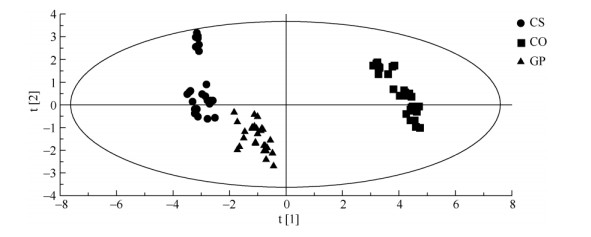
|
Fig. 2 Principle component analysis (PCA) of score plots derived from anatomic characteristics data of Camellia sinensis (CS), C. oleifera (CO) and grafted plants (GP). The principle components P1 and P2 represent 39.2% and 19.1% of the information derived from morphology anatomy, respectively. |
All of indexes, except of thickness of spongy tissue, were in order as C. sinensis < grafted plant < C. oleifera, because thickness of spongy tissue of grafted plant was close to that of C. sinensis. The thickness of palisade tissue and ratio of palisade tissue to spongy of grafted plant were higher than those of C. sinensis, and its leaf thickness was lower. Leaves could prevent moisture evaporation during drought with developed palisade tissue and simplified spongy tissue in mesophyll[15-16]. The thicknesses of upper and lower cuticle in grafted leaves were significantly bigger than those of C. sinensis. Therefore, grafted seedlings had high resistibility with strong protective covering. The ratio of palisade tissue to spongy tissue, fineness of leaf tissue and thickness of leaf epidermis had positive correlation with its cold resistance[17-18]. The results showed the thickness of upper and lower epidermis of grafted leaves not greatly increased compared to those of C. sinensis and obviously lower than that of stock (Fig. 3: B, F). However, the ratio of palisade tissue to spongy tissue and fineness of leaf tissue of grafted leaves were increased compared with those of C. sinensis(Fig. 3: I, J).
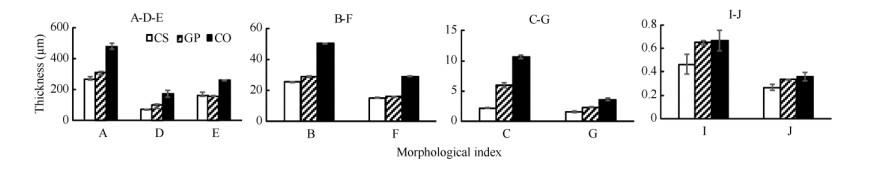
|
Fig. 3 Anatomical characteristics of leaves in Camellia sinensis (CS), C. oleifera (CO) and grafted plants (GP). A: Thickness of leaf; B: Thickness of upper epidermis; C: Thickness of upper cuticle; D: Thickness of palisade tissue; E: Thickness of spongy tissue; F: Thickness of lower epidermis; G: Thickness of lower cuticle; I: Ratio of palisade tissue to spongy tissue; J: Fineness of leaf tissue. |
In order to understand comprehensively about new grafted seedlings, the analysis of main meta-bolites was carried out. From Table 1, the content of caffeine accounted for almost 95% of the total purine alkaloids in C. sinensis leaves. However, little amount of caffeine was detected in C. oleifera leaves. The concentrations of 7-methylxanthine and theobromine were very low in leavesof C. sinensis, C. oleifera and grafted plants, respectively. Most importantly, the content of caffeine in grafted leaves (15.25 μmol g-1 FW) was much less than that of C. sinensis leaves (40.56 μmol g-1 FW). The content of purine alkaloids, especially caffeine was decreased significantly in grafted leaves in comparison to their scions, which might be due to the influence of their corresponding grafting stocks (C. oleifera). The grafted leaves contained much less purine alkaloids compared with those of C. sinensis leaves, which provide a possibility to obtain low caffeine variety of C. sinensis.
| Table 1 Contents (μmol g-1 FW) of purine alkaloids in leaves |
The contents of 18 free amino acids including theanine in leaves of C. sinensis, C. oleifera and grafted leaves were determined. Amino acids not only play an important role in the particular flavor of tea, but also show many healthy effects, such as promoting relaxation, inhibiting caffeine's negative effects, reducing blood pressure, and enhancing anti-tumor activity[19-21]. Previous studies showed that grafting obviously influenced amino acid content in new shootsof C. sinensis[22]. From Table 2, we could calculate the content of total amino acids in C. sinensis leaves was the highest, while the content of total amino acids in grafted leaves was almost reduced by half compared with that of C. sinensis leaves. And the content of total amino acids in C. oleifera leaves was the lowest. Among 18 free amino acids, we paid close attention to theanine, which is the most abundant amino acid and is the most one which is responsible for the specific flavor of green tea[23]. Theanine content in C. sinensis leaves (30.60 μmol g-1 FW) was more than twice in grafted leaves (14.10 μmol g-1 FW), while it was only trace amount in C. oleifera leaves (0.44 μmol g-1 FW).
| Table 2 Contents (μmol g-1 FW) of amino acids in leaves |
Flavonoids, including flavan-3-ols (catechins), anthocyanins, proanthocyanins flavonols, and phenolic acids, are derived from multiple branches of the phenylpropanoid biosynthetic pathways[24]. The major flavan-3-ols contain EC and EGC (non-gallate catechins), ECG and EGCG (gallate catechins). Catechins biosynthesis might not only exert anti-stress functions, but also are considered as part of primary metabolism[25]. The contents of flavan-3-ols and phenolic acids of grafted leaves were lower than those of C. sinensis (Table 3). Flavan-3-ols, especially EGC and GC, were the major components of flavonoids in leaves of three species. Compared with C. sinensis, non-gallate catechins (EC, C, GC, EGC) contents in grafted leaves were slightly increased except catechin (C). However, the contents of gallate catechins (ECG and EGCG) were decreased at various degrees in leaves of grafted plant, compared to those of C. sinensis. In addition, phenolic acids, including β-glucogallin (βG), gallic acid (GA) and theogallin (Gd), were not found in leaves of C. oleifera.
| Table 3 Contents (μmol g-1 FW) of flavan-3-ols, phenolic acids in leaves of Camellia |
Camellia sinensis and C. oleifera belong to Camellia but not in the same subgenus. Camellia sinensis is an evergreen, perennial cross-pollinated plant, belonging to the subgenusThea, while C. oleifera belongs to subgenusCamellia. In our research, the grafted seedlings withC. sinensis 'Shuchazao' as scions and wild oil tea (C. oleifera) in Dabieshan as stocks were cultivated. After morphology anatomy analysis, the leaves of grafted seedlings were more close to C. sinensis (the scions). Due to the thickness of palisade tissue and ratio of palisade tissue to spongy of grafted plant were higher than those of C. sinensis, it was indicated the grafted plant might be more adaptation to drought and high temperature environment than C. sinensis because of thick palisade tissue and unchanged spongy tissues derived from C. oleifera. The cuticle of leaf composed of unsaturated fatty acids has abilities of anti-diseases and anti-pests, reducing moisture evaporation, cold resistance, and so on[26].
After that, the contents of amino acids, purine alkaloids and polyphenols in leaves of grafted seedlings increased significantly compared with those of the stocks (C. oleifera), but they decreased obviously compared to the scions(C. sinensis). Among 18 free amino acids, theanine, the most abundant amino acid in tea leaves, is the most responsible for the specific flavor and taste of green tea[23]. Theanine content in C. sinensis leaves was more than twice in that of grafted leaves, while it was only trace amount in C. oleifera leaves. It was reported theanine is mainly synthesized in tea roots and then transported to shoots[27]. The theanine content decreased in grafted leaves probably due to the influence of the stocks (C. oleifera). And it was reported theanine is synthesized from glutamic acid and ethylamine by theanine synthetase (EC 6.3.1.6)[28], and ethylamine was produced from alanine in tea plants by alanine decarboxylase[29]. Low content of alanine in grafted leaves, might be another reason for less theanine biosynthesis in grafted tea seedlings. In the case of purine alkaloids, especially caffeine, it was decreased significantly in grafted leaves in comparison to the scions(C. sinensis). It was reported that caffeine biosynthesis existed in the young tea leaves, via a xanthosine→7-methyxanthosine→7-methylxanthine→theobromine→caffeine pathway. The reduction of caffeine in leaves of grafted seedlings might be due to the influence of their corresponding grafting stocks (C. oleifera), which may provide a prospect of variety improvement of low caffeine for C. sinensis. Compared with C. sinensis, non-gallate catechins (EC, GC, EGC) contents in grafted leaves were slightly increased. However, the contents of ECG and EGCG were decreased in leaves of grafted plant, compared to those of C. sinensis. These are probably due to less esterification, more hydrolysis or polymerization from simple catechins (EC and EGC) in the grafted tea seedlings. These results would lay a foundation for cultivation of C. sinensis and C. oleifera, and the grafted seedlings could be used to study the metabolism and transportation of specific secondary metabolites in C. sinensis.
| [1] | ZULAK K G, LISCOME D K, ASHIHARA H, et al. Alkaloids[M]//CROZIER A, CLIFFORD M N, ASHIHARA H. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet. Oxford: Blackwell, 2006: 102-136. doi: 10.1002/9780470988558.ch4. |
| [2] | SONG X H, SHI X G, LI Y Q, et al. Studies of cocoa tea, a wild tea tree containing theobromine[J]. Front Biol China, 2009, 4(4): 460-468. DOI:10.1007/s11515-009-0038-1 |
| [3] | ASHIHARA H, SANO H, CROZIER A. Caffeine and related purine alkaloids:Biosynthesis, catabolism, function and genetic engineering[J]. Phytochemistry, 2008, 69(4): 841-856. DOI:10.1016/j.phytochem.2007.10.029 |
| [4] | SAKATO Y. The chemical constituents of tea:Ⅲ. A new amide theanine[J]. Nippon Nogeikagaku Kaishi, 1949, 23: 262-267. |
| [5] | YAMAGUCHI S, NINOMIYA K. Umami and food palatability[J]. J Nutri, 2000, 130(4): 921S-926S. |
| [6] | LI Y F, HU L, WANG L H. Current status and prospect of researches and utilizations on Camellia oleifera resources[J]. Guangxi Agri Sci, 2009, 40(4): 450-454. DOI:10.3969/j.issn.2095-1191.2009.04.031 |
| [7] | WANG X N, CHEN Y Z, WU L Q, et al. Oil content and fatty acid composition of Camellia oleifera seed[J]. J CS Univ For Techn, 2008, 28(3): 11-17. DOI:10.3969/j.issn.1673-923X.2008.03.003 |
| [8] | HU J J, SHU Q L, CAO Z H, et al. Study on the chemical composition of shoot and leaf of Camellia oleifera elite varieties of different ecological regions in Anhui Province[J]. J NW Agri For Univ (Nat Sci), 2012, 40(6): 175-188. |
| [9] | WU S, LUO Y P. Variance and analysis of amino acid contents in grafted tea plants[J]. J Tea, 2000, 26(2): 75-77. DOI:10.3969/j.issn.0577-8921.2000.02.006 |
| [10] | CAO P R, YU X H, LI D, et al. Effects of different grafting ways on survival rate and growth of tea seedlings[J]. Guangdong Tea, 2011(5): 17-18. DOI:10.3969/j.issn.1672-7398.2011.05.006 |
| [11] | LEI Z G, HUANG Y F, HE H R. Study on Camellia oleifera and its germplasm resources[J]. Econom For Res, 2003, 21(4): 123-125. DOI:10.3969/j.issn.1003-8981.2003.04.041 |
| [12] | LI H P. Plant Microscopy Technique[M]. Beijing: Science Press, 2009: 85-90. |
| [13] | XU W P, SONG Q S, LI D X, et al. Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition[J]. Agric Food Chem, 2012, 60(28): 7064-7070. DOI:10.1021/jf301340z |
| [14] | JIANG X L, LIU Y J, LI W W, et al. Tissue-specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant (Camellia sinensis)[J]. PLoS One, 2013, 8(4): e62315 DOI:10.1371/journal.Pone.0062315 |
| [15] | MEI X Y, JIANG Z M, GAO S T, et al. Studies on anatomical structures of leaves of Juglans regia and Juglans sigillate varieties (excellent clones) and their drought resistances[J]. J NW For Coll, 1998, 13(1): 16-20. |
| [16] | CHEN R B. Observation on anatomical structure of tea leaves of different varieties[J]. Tea Sci Techn, 1989(3): 25-33. |
| [17] | QIN X J, LI F Y, HE J D, et al. The relations between the blade anatomical structure and characters of the new tea varieties in Guangxi[J]. Chin Agri Sci Bull, 2009, 25(10): 36-39. |
| [18] | WANG K L, HUANG X, LIU Q C, et al. Studies on the relationship between leaf structure and cold resistance of Camellia japonica L. (Naidong)[J]. J Qingdao Agri Univ (Nat Sci), 2007, 24(3): 189-192. DOI:10.3969/j.issn.1674-148X.2007.03.010 |
| [19] | KIMURA K, OZEKI M, JUNEJA L R, et al. L-theanine reduces psychological and physiological stress responses[J]. Biol Psychol, 2007, 74(1): 39-45. DOI:10.1016/j.biopsycho.2006.06.006 |
| [20] | SUGIYAMA T, SADZUKA Y. Theanine and glutamate transporter inhibitors enhance the antitumor efficacy of chemotherapeutic agents[J]. Biochim Biophys Acta, 2003, 1653(2): 47-59. DOI:10.1016/S0304-419X(03)00031-3 |
| [21] | YAMADA T, TERASHIMA T, KAWANO S, et al. Theanine, γ-glutamylethylamide, a unique amino acid in tea leaves, modulates neurotransmitter concentrations in the brain striatum interstitium in conscious rats[J]. Amino Acids, 2009, 36(1): 21-27. DOI:10.1007/s00726-007-0020-7 |
| [22] | WU S, LUO Y. Effect of grafting on the major biochemica1 components in the two-year-old grafted tea plants[J]. J Tea, 2001, 27(2): 22-26. DOI:10.3969/j.issn.0577-8921.2001.03.007 |
| [23] | YE M F, ZHOU H Z. Preliminary study on the quality and main biochemical variability of complex tea plants[J]. Newslett Seric Tea, 1996(1): 22-24. |
| [24] | WANG Y S, GAO L P, SHAN Y, et al. Influence of shade on flavonoid biosynthesis in tea[Camellia sinensis (L.) O. Kuntze][J]. Sci Hort, 2012, 141: 7-16. DOI:10.1016/j.scienta.2012.04.013 |
| [25] | AGATI G, TATTINI M. Multiple functional roles of flavonoids in photoprotection[J]. New Phytol, 2010, 186(4): 786-793. DOI:10.1111/j.1469-8137.2010.03269.x |
| [26] | ZHENG R, ZHOU F F, LIN P, et al. Anatomical characteristics of leaf transection of different cultivars of Camellia oleifera and their relationship analysis[J]. J Plant Resour Environ, 2013, 22(2): 18-29. DOI:10.3969/j.issn.1674-7895.2013.02.03 |
| [27] | DENG W W, OGITA S, ASHIHARA H. Ethylamine content and theanine biosynthesis in different organs of Camellia sinensis seedlings[J]. Z Naturforsch C, 2009, 64(5/6): 387-390. |
| [28] | SASAOKA K, KITO M, ONISHI Y. Some properties of the theanine synthesizing enzyme in tea seedlings[J]. Agri Biol Chem, 1965, 29(11): 984-988. DOI:10.1080/00021369.1965.10858501 |
| [29] | TAKEO T. L-Alanine as a precursor of ethylamine in Camellia sinensis[J]. Phytochemistry, 1974, 13(8): 1401-1406. DOI:10.1016/0031-9422(74)80299-2 |
 2017, Vol. 25
2017, Vol. 25


